The role of pulmonary vein isolation in patients with non-paroxysmal atrial fibrillation (AF) is only modest. Several studies have demonstrated the role of the left atrial appendage (LAA) in initiating and maintaining of this arrhythmia. We review in this article the incremental benefit in free-arrhythmia recurrence of LAA electrical isolation in patients undergoing procedures for persistent AF or long standing persistent AF either using radiofrequency ablation, cryoablation or Lariat device implantation. Likewise, acute complications, anticoagulation and the risk of ischemic stroke after LAA electrical isolation (LAAEI) are analyzed. LAAEI in addition to standard ablation appears to have a substantial incremental benefit to achieve freedom from all atrial arrhythmias in patients with persistent AF and long standing persistent atrial fibrillation (LSPAF) without increasing acute procedural complications and without raising the risk of ischemic stroke.
Atrial fibrillation (AF) is the most common arrhythmia in the elderly population (>65 years). Several antiarrhythmic medications have been used to control this arrhythmia with limited efficacy and numerous short and long-term side effects. Likewise, several arrhythmogenic mechanisms have been proposed for AF and in turn many ablation strategies have been tested. Most of these studies have been small observational single center experiences, which have not been validated or replicated worldwide. As elegantly demonstrated in the landmark study by Haissaguerre and colleagues, pulmonary veins (PV) play an extremely important role in the initiation and maintenance of AF.1 It has been established by several studies that wide antral pulmonary vein isolation (PVI) is more efficacious than ostial PVI in achieving freedom from any atrial tachyarrhythmia recurrence at long-term follow-up.2 It is now well known that the role of the PVs is more pronounced in paroxysmal AF than in persistent AF or long-standing persistent AF (LSPAF). Persistent AF or LSPAF is a complex arrhythmia and frequently requires extensive catheter ablation (CA) targeting thoracic veins other than the PVs and some times large areas of atrial myocardium. Several studies reporting results on CA of persistent AF have been published to date. Most of these studies are observational with very different methodologies and end points using different ablation strategies and targets. As a result, there has been an astonishing variation in success rates at short- and long-term follow-up within and between techniques, suggesting that the optimal ablation approach for persistent AF and LSPAF is still to be elucidated.3
Although the STAR AF (Substrate and Trigger Ablation for Reduction of Atrial Fibrillation) trial was the first randomized control trial (RCT) showing that complex atrial fractionated electrograms (CFAEs) ablation guided by automated mapping software could have an additive benefit over PVI alone,4 more recently the results from the STAR AF II trial have been published.5 In this large multicenter RCT, participants with persistent AF received PVI vs. PVI plus CFAEs ablation vs. PVI plus empiric linear ablation (i.e. atrial roof line and a line along the mitral valve isthmus). Surprisingly, after following these patients for 18 months, freedom from AF occurred in 59% of the PVI group, 48% of the PVI plus CFAEs group, and 44% of the PVI plus lines group with no statistical difference among groups (p=0.15). Freedom from AF after two procedures occurred in 72% of the PVI group, 60% of the PVI plus CFAE group, and 58% of the PVI plus lines group (p=0.18).5 Empirical isolation of the posterior left atrial (LA) wall might also have a role in patients undergoing AF ablation. From an embryologic, anatomic, and electrophysiological standpoint, the posterior wall should be considered an extension of the PVs and its isolation has been proven to improve outcomes in paroxysmal and non-paroxysmal AF patients.6 We recently published data from the AATAC multicenter RCT, in which patients with persistent AF and left ventricular ejection fraction <40% were randomly assigned to undergo CA for AF or receive amiodarone.7 After a minimal follow-up of 24 months, 70% of patients in RFA group were recurrence free after average 1.4 procedures in comparison with 34% in the amiodarone group, p<0.001. More importantly, higher success was reported in patients undergoing PVI and posterior wall isolation in comparison with PVI alone (79% and 36%, respectively, p<0.001). There was no significant difference between PVI alone and amiodarone, which suggest that posterior wall isolation might be necessary to achieve better results.7 However, there is no large RCT proving this concept.
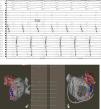
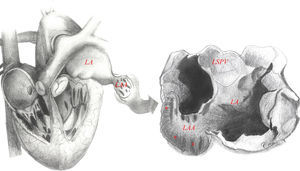
Left atrial appendageEmbryologically, the left atrial appendage (LAA) is a remnant of the primordial embryonic LA, which explains its trabecular appearance (Figure 1). It was not until the mid-1950s that the LAA, previously considered a trivial and non-functional anatomic cardiac structure, was identified as the main location of thrombus formation in AF (i.e., 93% in non-valvular AF).8 Ever since, the LAA has become the interest of several investigators who have looked deeper into it in different angles including understanding its anatomy, physiology and role on initiation and maintenance of AF.
Left atrial appendage anatomy (classic ‘chicken wing’ morphology). The LAA is a remnant of the primordial embryonic left atrium, hence the presence of pectinate muscles. Note the close relationship between the LAA and the LSPV, which is very important in the setting of LAAEI during ablation for AF. Asterisks show heavy trabeculations (pectinate muscles). Abbreviations: LA: left atrium; LAA: left atrial appendage; LSPV: left superior pulmonary vein.
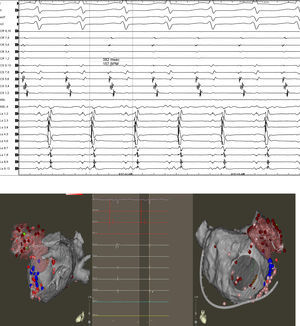
We always believed that this structure deserved special consideration when mapping and ablating patients with persistent AF and LSPAF. In 2005, Takahashi et al. reported a case of a patient with paroxysmal AF, in whom multiple foci were identified in the LAA after PVI. The patient was successfully treated by CA by disconnecting this structure from the LA.9 The arrhythmogenic role of the LAA was not well known until 2010 when our group initially reported on the prevalence of triggers firing from the LAA and the optimal strategy to eliminate these foci to increase the procedural success rate. Nine hundred eighty-seven consecutive patients underwent redo CA for AF (29% paroxysmal, 71% non-paroxysmal) in that study. All patients had demonstrated isolated PVs. The study not only revealed that 27% of patients had firing from the LAA, but also that in 8.7% the LAA was the only source of arrhythmia with no PV or other extra-PV site reconnection. More importantly, complete isolation of the LAA showed an arrhythmia recurrence of only 15% at 12-month follow-up compared to 68% and 74% when focal ablation of LAA triggers or no ablation was performed, respectively (Figure 2).10 Hocini et al., have also reported on the fact that the LAA is an important source of localized ectopic triggers and reentrant atrial tachycardia in patients with persistent AF during first and repeat ablation procedures.11
Top: Atrial tachycardia originating in the LAA with a TCL of 382 ms (15 bpm). Bottom: Electroanatomic mapping (left lateral and anteroposterior views) of the LA depicting a Lasso and ablation catheter in the LAA. Intracardiac electrograms depicting significant atrial activation delay into the LAA. The first beat shows the near and far field. The second beat illustrates the moment when the LAA is isolated (loss of neat field electrogram).
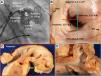
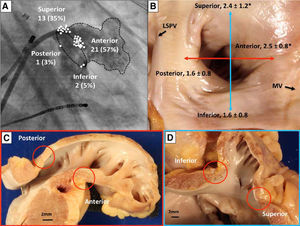
Because the LAA has a very thin wall and may be prone to perforation, LAA electrical isolation (LAAEI) is currently performed by delivering radiofrequency (RF) energy at the level of the LAA ostium, which is considerably thicker. Panikker demonstrated in cadaveric hearts that the thickest LAA ostial areas requiring longer RF ablation are the anterior (2.5±0.8 mm [range, 1.4-4.0 mm]) and superior (2.4±1.2 mm [range, 1.1-4.8 mm]) edges (Figure 3).12 LAAEI is usually guided by intracardiac echocardiography and 3-dimensional mapping systems using a circular mapping catheter. To obtain electrical isolation in this structure, up to 30 minutes of high power RF ablation are required in some cases. The RF settings during LAAEI include power up to 43 Watts, while maintaining a catheter tip temperature of 42°C for a maximum of 60 seconds per ablation site (Figure 4).
Sites of acute LAA reconnection and regional variation in LAA ostial thickness. (A) Location of acute LAA reconnection sites superimposed on right anterior oblique (35°) fluoroscopic view of the LA with contrast opacified LAA. All of the reconnection sites are located at the base of the LAA. The location of each reconnection is marked with a circle on the segment of LAA involved. (B) Gross view of non-ablated LAA, LSPV, and mitral valve. The LAA walls were cut longitudinally along the red and blue lines, and the regional LAA wall thickness was measured. Asterisks indicate significantly thicker ostial tissue at superior and anterior LAA margins compared with inferior and posterior margins (p=0.02). Sections through anterior and posterior walls (C) and superior and inferior walls (D). Note the close proximity of the left circumflex coronary artery to the anterior wall of the LAA. Adapted from Circ Arrhythm Electrophysiol. 2016;9:e003710.
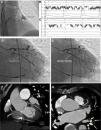
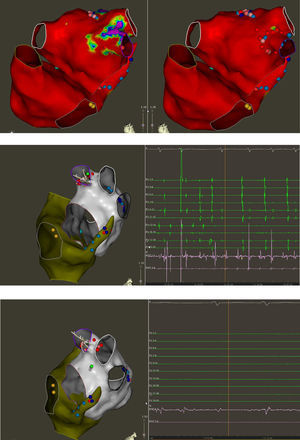
Top: Electroanatomic voltage map of a 79-year-old patient with ischemic cardiomyopathy who underwent CABG, cox-MAZE procedure and LAA ligation in 2011 for persistent AF. Patient presented to CCU with decompensated heart failure and AF with rapid ventricular response. Voltage map revealed severe right and left atrial scarring. Pre (left) and post (right) LAAEI. Note RF ablation lesions delivered at the ostium of the LAA. Middle: PentaRay catheter is in the LAA while delivering RF energy. Note that AF terminates into normal sinus rhythm. Bottom: PentaRay catheter is in the LAA showing no signals demonstrating LAAEI.
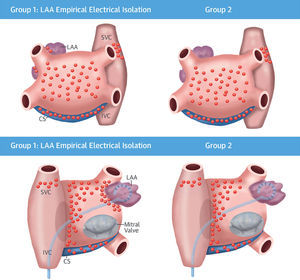
The results of the effect of empirical LAAEI on long-term procedure outcome in patients with LSPAF undergoing CA (i.e. the BELIEF study) have been recently published.13 This RCT compared the ablation outcome in patients with LSPAF undergoing LAAEI plus our standard approach (i.e., PV isolation, posterior wall isolation, roof and anterior septum ablation, SVC isolation and non-PV triggers), versus our standard approach alone (Figure 5). At 12 months follow-up, 56% in the group with LAAEI and 28% of patients in the control group were recurrence free after a single procedure (p=0.001). During repeat procedures, cumulative success at 24-month follow-up was reported in 76% of patients who underwent LAAEI and 56% of patients in the control group (p=0.003). This RCT demonstrated that both after the first and redo procedures in patients with LSPAF, empirical LAAEI improved long-term freedom from atrial arrhythmias without increasing complications.13 Panikker et al. recently evaluated the feasibility, safety, and efficacy of LAAEI in patients undergoing CA of persistent AF. The authors showed that freedom from AF/atrial tachycardia at 12-month follow up after a single procedure off antiarrhythmic drugs was significantly higher in the LAAEI group (95%) than in the control group, who received only standard CA (63%) (i.e. PVI, roof and posterolateral mitral lines and CTI line), p=0.036.12 Similarly, Park and collages reported their experience of LAAEI following extensive LA ablation of the anterior and posterolateral walls (i.e. LA anterior wall, perimitral isthmus line) in patients with persistent AF. The group of patients in whom LAAEI was obtained had a significantly higher success rate at 21 months follow-up when compared with the control group (83% vs. 50%, p=0.028).14
This BELIEF trial randomized patients with LSPAF to standard ablation plus non-PV triggers plus empirical LAAEI (left) versus standard ablation plus non-PV triggers ablation (right). (Top) Lesion set in anteroposterior view. (Bottom) Lesion set in posteroanterior. Adapted from J Am Coll Cardiol 2016;68:1929–40.
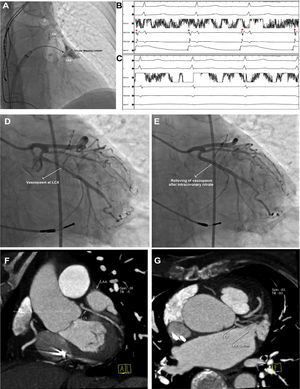
The FIRE and ICE RCT determined that cryoballoon ablation is non-inferior to RF ablation in patients with paroxysmal AF.15 However, the applicability of cryoablation for non-PV sites was thought to be limited. Nevertheless, due to safety concerns regarding RF ablation of a thin-wall LAA, some centers around the world have opted to perform empirical LAAEI using cryoenergy. Besides standard PVI in all patients with persistent and LSPAF the 28-mm third-generation cryoballoon (CB-Advance Short Tip, Medtronic, Minneapolis, Minnesota) and the inner lumen circular mapping catheter (Achieve 15 mm, Medtronic) are advanced into the LAA. Subsequently, the cryoballoon is inflated and positioned at the LAA ostium aiming for complete occlusion (Figure 6).16 Using this technique the left phrenic nerve, because of its proximity to the LAA, is monitored by fluoroscopy during spontaneous breathing if procedure is being performed during conscious sedation or by high output pacing during general anesthesia. Coronary angiogram during or immediately after cryoablation is usually performed to rule out vasospasm of the proximal segment of the circumflex artery (Figure 6). Vasospasm should be suspected if patients develop symptoms or signs of myocardial ischemia during LAAEI.
Complete occlusion of the LAA using cryoballoon (A). During cryoablation, a progressive LAA spike (B) delay resulted in LAA isolation (C). Coronary angiogram after cryoablation revealed a vasospasm at the proximal segment of the circumflex artery (D), which has been relieved by administration of intracoronary nitrate (E). Analysis of the pre-ablation computerized tomography raw data showing the close proximity of LCX with LAA ostium (F, G). *: LAA electrogram.
Our findings in the BELIEF trial, showing the critical role of the LAA, are corroborated in a recent well-designed study by Yorgun et al. using cryoablation instead or RF ablation for empirical LAAEI.17 Their results are clinically relevant due primarily to the fact that the authors have also demonstrated the beneficial role of empirical LAAEI, not only on patients with LSPAF, but mostly in patients with persistent AF. In this study, the authors sought to evaluate the safety and efficacy of empiric cryoballoon LAA isolation as an adjunct to PVI compared to the PVI-only strategy. Interestingly, this was a propensity-score matched study, which is almost as good as a RCT, as it is evidenced by no significant differences between the experimental and control groups including comorbidities, echocardiographic parameters, antiarrhythmic medications and anticoagulants. At the 12-month follow-up, 67% of patients in the control group and 86% of patients in the LAAEI group were free of atrial arrhythmias (p<0.001). This study also clearly demonstrated that this cryoablation might have a larger role in treating patients with non-paroxysmal AF. Likewise, despite the fact that cryoablation has been criticized due to the need for longer fluoroscopy time, the authors were able to perform PVI and LAA using less than 10 minutes of fluoroscopy in most cases in a very efficient manner.17 The authors constantly paced the left phrenic nerve from the LAA using circular mapping catheter throughout the freezing cycle. Furthermore, left coronary angiography was performed simultaneously or after the LAAEI to exclude the left circumflex artery vasospasm. One patient experienced left phrenic nerve paralysis, which resolved the day after the procedure. Asymptomatic left circumflex artery spasm was observed in 4% of the LAAEI group, which was completely resolved after administration of intracoronary nitrates. Localization of phrenic nerve from the LAA and left coronary angiography should be routinely implemented in future studies using RF and Cryoenergy ablation to isolate the LAA.
In lieu of no optimal ablation strategy for patients with persistent AF and LSPAF, the BELIEF trial13 and the study by Yorgun et al.17 have become landmark studies since they demonstrated in a very clean fashion, either using RF ablation or cryoablation, that empiric LAAEI might be indeed necessary in addition to PVI to improve clinical outcomes in this population. As we have learned for empiric isolation of PVs, empirical ablation of the LAA is also important and can be performed without clear evidence of triggers from this structure. With the negative results from the STAR AF II study, CFAEs or empiric linear ablation are no longer recommended.5 Likewise, there is also limited data supporting the use of posterior wall isolation for these patients. A meta-analysis with five studies with poor methodology demonstrated a small benefit, which was largely driven by the decreased AF recurrence.6 Recurrence rates of atrial tachycardia/flutter were comparable between groups.6
LARIAT device for LAA electrical isolationPreclinical models and surgical studies postulated that the LAA could be potentially electrically isolated by surgical epicardial clipping occlusion. One group demonstrated complete electrical isolation of the LAA in 10 AF patients who underwent off-pump coronary artery bypass surgery with bilateral PVI and LAA clip occlusion. Before and after the clip was placed, pacing maneuvers were performed to assess electrical exit and entry blocks from the LAA.18 Han and colleagues studied 68 patients who underwent LAA ligation with the LARIAT device and demonstrated a significant decrease in LAA unipolar and bipolar voltages before and after the snare was tightened (Figure 7). Ninety four percent of these patients had a reduction in LAA voltage with a third of these having complete elimination of LAA voltage. Pacing from the LAA after closure of the snare demonstrated complete isolation of the appendage with no capture of LA in 90% of the patients.19 This may be consistent with LAA ischemic necrosis. Whether or not LAA ligation might play an important role in decreasing the AF burden was evaluated by the LAALA-AF registry.20 Lakkireddy et al. conducted a prospective observational study to estimate the benefit of concomitant LARIAT procedure when added to conventional ablation of persistent AF. The primary outcome of freedom from AF at 1 year off antiarrhythmic therapy after 1 ablation procedure was higher in the LARIAT group (65% vs. 39%; p=0.002). More patients in the ablation-only group underwent repeat ablation because of AF recurrence (33% vs. 16%; p=0.018).20 Furthermore, the benefit in decreasing the recurrence of AF following AF ablation after LARIAT placement is currently under investigation in the multicenter randomized aMAZE trial (LAA Ligation Adjunctive to PVI for Persistent or Longstanding Persistent Atrial Fibrillation NCT02513797).
LAA ligation/excision during cardiac surgerySurgical LAA occlusion or excision has been performed as an alternative treatment option to prevent embolic strokes in patients undergoing cardiac surgery. In 1949 Madden achieved the first LAA excision in two patients with AF and rheumatic mitral disease. Thereafter, a randomized trial, the LAA occlusion study (LAAOS), was conducted in 77 patients to evaluate the safety and efficacy of occlusion at the time of elective coronary bypass graft surgery using sutures and staples. Occlusion was achieved in only 66% of the patients and the use of staples had the highest efficacy assessed. Staples had 72% efficacy and sutures only 45%.21 A meta-analysis of clinical trials demonstrated that most studies reported merely 55-66% successful occlusion rate using a variety of methods including stapling, ligation and amputation, hence revealing a significant limitation of the surgical approach. A proposed reason for this might be inability to achieve acceptable rates of complete occlusion on TEE during the procedure.22 A prospective study in 2008 evaluated the success of several surgical LAA closure techniques by transesophageal echocardiography (TEE). In general, there was a high rate of failure (60%), with LAA excision being the most effective technique with a 73% successful rate out of the successful LLA closures. Success rate for suture and stapler exclusion was 23% and 0%, respectively.23 In 2010, the Food and Drug Administration (FDA) approved the AtriClip for stroke prevention in patients with AF undergoing open-heart surgery. Yet, data on this device is scarce. Most of these patients are left with an LAA stump, which remains arrhythmogenic and requires endocardial electrical isolation.24Figure 4 illustrates a case a patient with persistent AF with triggers originating at the stump of a previously ligated LAA.
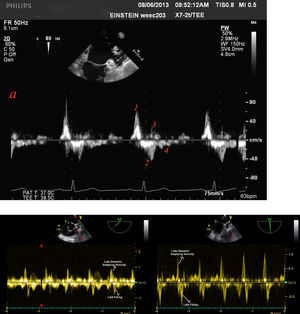
Anticoagulation after LAA electrical isolationLAAEI might cause electromechanical dissociation with the potential for thrombus formation. Two parameters, LA dilatation and low LAA emptying and filling velocities have remarkably been proven to be predisposing factors for smoke and thrombus formation.25 The LAA is a highly mobile and dynamic structure with distinct patterns of contraction and relaxation either in sinus rhythm or in AF. During sinus rhythm four waves are noted. The first wave starts in late diastole and represents rapid forward flow probably due to LAA contraction/emptying, this is followed by a second large retrograde wave that represents LAA filling. Then, additional forward and retrograde wave velocities are seen. These might be the result of ventricular relaxation and LAA elasticity, respectively (Figure 8A).26 It is important to recognize that these velocities are heavily dependent on loading conditions and left ventricular function.27 In our current practice TEE is performed six months post LAA ablation to assess the presence of a consistent A wave, LAA flow velocity, and LAA contractility. Patients with poor LAA velocity (<0.4 m/s) and/or abnormal LAA function are maintained indefinitely on oral anticoagulation regardless of the CHA2DS2-VASc score. In patients who remain in sinus rhythm with CHA2DS2VASc score of <2 in the LAA isolation group, and with adequate LAA flow velocity (>0.4 m/sec) and minimal smoke (Grade I-II) at the 3-month follow-up oral anticoagulation can be discontinued. In the BELIEF trial, no stroke or transient ischemic attack was reported in the LAAEI group, whereas 4 (4.5%) patients had stroke in the standard ablation group 2 (p=0.12).13 Similar results were observed by Yorgun et al. who used Cryoenergy for LAAEI. This study reported the LAAEI group only one ischemic stroke. This complication was observed five months after the procedure in a patient with CHA2DS2VASc score of 5, who had discontinued anticoagulation therapy for 10 days. Otherwise, LAAEI did not cause any other thromboembolic events despite the decrease in LAA flow velocities during the follow-up.17 Interestingly, although the LAA flow velocity usually decreases after isolation some patients maintain velocities within normal ranges (Figure 8B). Although, it seems counterintuitive that even though the LAA is electrically isolated, this structure can still contract. The exact mechanism of this phenomenon still remains to be elucidated. Rillig and colleagues28 reported that electrical isolation of LAA is associated with an increased incidence of LAA thrombus and stroke when compared to patient that did not undergo LAAEI. Nevertheless, all three patients who had a cerebral thromboembolic event had AF recurrence with one patient off NOAC, one patient on warfarin with subtherapeutic international normalized ratio at the time of the event, and one patient on dabigatran without verification of patient compliance. Moreover, that group also performed a more extensive approach to isolate the LAA with a wider area of ablation after PV isolation, which consisted in a posterolateral mitral isthmus line and an anterior line from the right superior pulmonary vein to the lateral mitral annulus in the majority of patients. This type of extensive ablation might have compromised the overall atrial contraction and could have become prothrombotic.28 We have reported data from 1854 consecutive AF patients receiving LAA isolation along with PVI. TEE at six months post-ablation follow-up showed impaired LAA mechanics in 58% of the patients. The overall thromboembolic event rate was 0.08% and 2.26% in on- and off-anticoagulation groups, respectively (p<0.001). Of the 14 patients with stroke, 12 (85.7%) had subtherapeutic warfarin level or discontinued their NOACs for more than 5 days. These results provide strong evidence that LAA isolation is not associated with a higher risk of thromboembolic events even in the presence of impaired LAA function as long as optimal oral anticoagulation is rigorously taken.29
Blood flow velocities measured by transoesophageal echocardiography in normal sinus rhythm. Top: Four distinct waves are seen. The first wave (1) starts in late diastole and represents rapid forward flow (0.62 m/s) probably owing to LAA contraction or emptying, followed by a second (2) large retrograde wave (0.42 m/s) that represents LAA filling. Two additional forward (3) and retrograde (4) wave velocities are then seen. These might be the result of ventricular relaxation and LAA elasticity, respectively. Bottom: Emptying and filling LLA velocities prior (left) and 6 months following radiofrequency ablation (right) of this structure in a patient with LSPAF. Note that velocity remained the same at 1 m/s.
Finally we would like to conclude saying that empirical LAAEI in addition to PVI appears to be the best strategy to achieve freedom from AF in patients with persistent AF and LSPAF and that anticoagulation management of these patients should be better addressed.
DisclosuresDr. Di Biase is a consultant for Biosense Webster, Stereoataxis, Boston Scientific and Abbott. Dr. Di Biase received speaker honoraria/travel from Medtronic, Atricure, EPiEP and Biotronik. Natale received speaker honoraria/travel from Medtronic, Atricure, EPiEP Biotronik and Janssen. Dr. Romero has no disclosures.