Spontaneous echo contrast (SEC) is the appearance of swirling, smoke-like echoes in the left atrium (LA) and is accepted as an independent predictor of thromboembolic risk. There is an established relationship between the inflammatory state and the prothrombotic state. Therefore, we investigated the relationship between the Systemic Immune Inflammation Index (SII), a new inflammation parameter introduced recently, and SEC in patients with mitral stenosis (MS).
Material and methodsA total of 262 patients who underwent percutaneous mitral valvuloplasty (PMBV) for MS were included in this study. The patients were divided into two groups: patients with MS complicated by SEC and patients with MS without SEC, based on whether SEC occurred in the LA.
ResultsThere were 79 patients (mean age 47.1 ± 6.6, 30.3% male gender) in the SEC (+) group, while there were 183 patients (mean age 46.4 ± 8.6, 29.5% male gender) in the SEC (-) group. In multivariate analysis, high levels of SII were an independent risk factor for SEC in patients with MS (OR: 1.001, 95% confidence interval (CI): 1.000-1.001, p<0.001) together with high levels of C-reactive protein (OR: 1.145, 95% CI: 1.027-1.277, p=0.014). The receiver operating characteristics (ROC) curve analysis showed that at a cutoff value of 547.6 for SII to predict SEC with 74.6% sensitivity and 77.6% specificity (area under ROC curve=0.736 (95% CI: 0.668-0.805), p<0.001).
ConclusionOur study showed that the SII levels were independently associated with SEC in patients with MS.
O contraste espontâneo (CE) consiste no aparecimento de ecos em espiral semelhantes ao fumo de tabaco na aurícula esquerda sendo aceite como um fator preditivo independente de risco tromboembólico. Existe uma relação estabelecida entre o estado inflamatório e o estado protrombótico. Portanto, analisámos a relação entre Índice de Inflamação Imunológica Sistémica, (IIIS), um novo parâmetro recentemente introduzido e o CE em doentes com estenose mitral (EM).
Material e métodosForam incluídos neste estudo 262 doentes, submetidos a valvuloplastia mitral percutânea (VMP) por EM. Os doentes foram divididos em dois grupos: em doentes com EM complicada por CE e em doentes com EM sem CE, com base na ocorrência de CE na aurícula esquerda.
ResultadosForam estudados 79 doentes (idade média 47,1 ± 6,6, 30,3% do sexo masculino) no grupo CE (+) e 183 doentes (idade média 46,4 ± 8,6, 29,5% do sexo masculino) no grupo CE (-). Na análise multivariada, níveis elevados de IIIS constituíram um fator de risco independente de CE em doentes com EM (OR: 1,001, intervalo de confiança 95% (IC): 1,000-1,001, p<0,001) juntamente com níveis elevados de PCR (OR: 1,145, IC 95%: 1,027-1,277, p=0,014). A análise da curva das características de funcionamento do recetor (ROC) mostrou que com um valor cut-off de 547,6 para IIIS é fator preditivo de CE com 74,6% de sensibilidade e 77,6% de especificidade (área sob curva ROC=0,736 (IC 95%: 0,668-0,805), p<0,001).
ConclusãoO nosso estudo mostrou que os níveis de IIIS associam-se independentemente a CE em doentes com EM.
Spontaneous echo contrast (SEC) is the appearance of blood that normally does not show enhancement echocardiographically, gaining spontaneous echogenicity, such as typical rolling smoke on echocardiography.1
This image is seen the most in the left atrium (LA) and is often caused by left ventricular systolic and/or diastolic dysfunction accompanied by mitral stenosis (MS), nonvalvular atrial fibrillation (AF), prosthetic mitral valve, and left atrial enlargement.2,3
Studies have revealed that SEC is an independent risk factor for thrombus formation and systemic embolization in the LA, independent of AF and MS.4,5 It has been reported that SEC is also predictive of future thrombus formation and stroke development.6
Although the pathophysiology of SEC is still not fully understood, studies have shown that inflammation and the increase in various inflammatory markers such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) are closely related to the formation of SEC.7–9
The Systemic Immune Inflammation Index (SII), a new recently introduced inflammation parameter, is a robust prognostic indicator of adverse outcomes in various cardiovascular diseases.10,11 There has been no study on the relationship between this new parameter, which is obtained by dividing the product of the neutrophil (N) number and platelet count by the number of lymphocytes (L), with the development of SEC. This study investigated the relationship between SEC and SII levels detected by transesophageal echocardiography (TEE) in patients with mitral stenosis.
MethodsStudy populationA total of 262 patients who underwent percutaneous mitral valvuloplasty (PMBV) for MS at our clinic between January 2013 and December 2020 was included. The study plan was approved by the institutional ethics committee and conducted following the Helsinki Declaration.
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) were performed in each patient to exclude thrombus formation in the LA before PMBV. The presence of SEC for all patients was determined by TEE. The study population was divided into two groups: patients with MS complicated by SEC and patients with MS without SEC, based on whether the SEC occurred in the LA.
Patients with LA thrombus, patients with heart failure (ejection fraction <50%), history of malignancy, connective tissue disease, thyroid disease, history of the hematologic disease, active infection, chronic inflammatory disease, steroid use, severe renal insufficiency (glomerular filtration rate <30 ml/min/1.73 m2) or liver failure (alanine aminotransferase >60 IU/L) were excluded.
Classic cardiovascular risk factors such as age, gender, diabetes, hypertension, dyslipidemia and smoking, medical history data, and admission laboratory findings of 262 patients were analyzed in detail. Before TTE, blood pressure measurements were performed in all patients by trained auxiliary health personnel. Heart rate evaluation was performed simultaneously with blood pressure measurement. The presence of AF was determined by an electrocardiogram.
Laboratory measurementsImmediately after the TEE procedure for laboratory analysis, antecubital venous blood samples were drawn into tripotassium EDTA anticoagulated tubes. Hemoglobin, platelet, white blood cell counts, neutrophils, lymphocytes, high sensitivity C-reactive protein (CRP), lipid profile, and all routine biochemical tests were performed on an autoanalyzer (Roche Diagnostic Modular Systems, Tokyo, Japan). Samples were studied immediately to prevent platelet swelling.
The neutrophil-lymphocyte ratio was found by dividing the number of neutrophils by the number of lymphocytes. The platelet-lymphocyte ratio (PLR) was found by dividing the platelet count by the number of lymphocytes. A new inflammatory index, SII, was obtained by dividing the product of the neutrophil count and the platelet count by the lymphocyte count.
Echocardiography examinationAll echocardiographic examinations were performed after at least 15 minutes of rest, in the left lateral position (two-dimensional, M-mode, color Doppler echocardiography) using the Vivid 7 pro ultrasound system (Vivid 7 pro, GE, Horten, Norway, 2-4 MHz phased array transducer ultrasound system). Conventional echocardiographic images were obtained from parasternal and apical images according to the guidelines of the American Echocardiography Association.12 TTE and TEE were performed at the same time.
Simpson's rule was used to calculate LV ejection fraction. Mitral valve area was calculated from the parasternal short axis using the planimetric method. The mitral valve pressure difference was calculated by taking the average of the pressure differences measured using the Doppler flow method. LA volume and the diameters of the right heart chambers were measured. Pulmonary artery pressure (PAP) measurement was taken on tricuspid regurgitation. The trans-tricuspid gradient was calculated using the maximum velocity of the tricuspid regurgitation jet, and the estimated right atrial pressure (10 mmHg) was added to the trans-tricuspid gradient to obtain the estimated systolic PAP.
After local anesthesia was applied to the patient's throat with topical 10% lidocaine spray, TEE was performed. TEE images were obtained in the most visible position after the TEE probe was advanced 25-35 cm in the esophagus. After standard images of the heart cavities and valves were taken, the LA and left atrial appendage (LAA) were examined in detail for SEC and thrombus. All images were evaluated by two experienced independent echocardiographer cardiologists who were unaware of other data and results of the patients. SEC severity, graded according to the criteria determined by Fatkin et al. from 0 to 4 in terms of density, mobility, and localization.13 SEC rating was as follows: 0, No SEC, 1+ (mild); temporarily seen during the cardiac cycle; minimal echogenicity located in the LAA or rarely in the LA, 2+ (mild-moderate); same distribution as a first degree but more intense echogenicity; 3+ (moderate); SEC image covering the entire left atrial body and appendix, which is seen constantly with varying intensity throughout the cardiac cycle, 4+ (severe), LAA appears with intense dark echo density and makes a very slow rotational motion, presence of echo density appearance in the same intensity in the LA. The intra- and interobserver variability were assessed from randomly selected 100 patients. The intra- and inter-observer variability for the presence of SEC was less than 3%. Both the intra- and inter-observer variability for 1(+) SEC were 3%, for 2(+) SEC, the intra- and inter-observer variability were 4% and 3%, respectively; for 3(+) SEC, the corresponding values were 2% and 3%, respectively; and for 4(+) SEC, both intra- and inter-observer variability were 2%.
Statistical analysisStatistical analyses were performed using SPSS version 21.0 (SPSS Inc, Chicago, IL, USA) software for Windows. The distribution of quantitative variables was checked with the Shapiro Wilk test. Descriptive data were given as mean ± standard deviation and median (interquartile range (IQR)), depending on normality of distribution. Median and IQR were given when the variable did not follow a normal distribution. The independent samples t-test was used to compare normally distributed quantitative variables, and the Mann-Whitney U test was used to compare non-normally distributed quantitative variables. Categorical variables were compared with the chi-square test. Categorical variables are all shown as percentages or numbers. A one-way analysis of variance test was used to compare the SII values in the SEC (+) group. The effects of different variables on the development of SEC were calculated with univariate analysis. For multivariate regression analysis, parameters with a p<0.10 in univariate analysis were included in the model. The cutoff level of the SII, NLR, and CRP in predicting SEC formation was determined by performing receiver operating characteristic curve analysis. The area under the curve (AUC) values of each parameter mentioned were compared with the Delong test in the Medcalc version 19.6.4 test version statistics program (MedCalc Software Ltd, Ostend, Belgium).14 p values lower than 0.05 were considered to demonstrate statistical significance.
ResultsA total of 262 patients who underwent PMBV for MS were included in the study. The patients were divided into SEC (+) and SEC (-) according to the presence of SEC. There were 79 patients (mean age 47.1 ± 6.6, 30.3% male gender) in the SEC (+) group, and 183 patients (mean age 46.4 ± 8.6, 29.5% male gender) in the SEC (-) group. The primary demographic characteristics, comorbidities, and medications of the study groups are presented in Table 1. There was no difference between the two groups regarding mean age and gender (p=0.484, p=0.887, respectively). There was no significant difference between the groups regarding diabetes, hypertension, previous coronary artery disease and history of cerebrovascular disease, presence of atrial fibrillation, smoking status, and body mass index (Table 1). Although the history of peripheral embolism was observed with a higher rate in the SEC (+) group, it was not statistically significant (p=0.151). Aspirin and warfarin drug use was also not different between the groups (p=0.320, p=0.123, respectively). When looking at hemodynamic parameters such as heart rate, systolic and diastolic blood pressure, there was no significant difference between the SEC (+) and SEC (-) patients (Table 1).
Demographic characteristics of the study populations.
| Spontaneous echo contrast | |||
|---|---|---|---|
| SEC (+) | SEC (-) | p value | |
| Variables | (n=79) | (n=183) | |
| Age (years) | 47.1 ± 6.6 | 46.4 ± 8.6 | 0.484 |
| Men gender (n, %) | 24 (%30.3) | 54 (%29.5) | 0.887 |
| Diabetes Mellitus (n, %) | 7 (%8.8) | 29 (%15.8) | 0.132 |
| Hypertension (n, %) | 14 (%17.7) | 47 (25.6) | 0.162 |
| Coronary artery disease (n, %) | 3 (%3.7) | 19 (%10.3) | 0.078 |
| Peripheral embolic events (n, %) | 7 (%8.8) | 8 (%4.3) | 0.151 |
| Cerebrovascular disease (n, %) | 1 (%1.2) | 4 (%2.1) | 0.617 |
| Atrial fibrillation (n, %) | 33 (%41.7) | 59 (%32.2) | 0.133 |
| Current smoker (n, %) | 6 (%7.5) | 18 (%9.8) | 0.564 |
| Systolic blood pressure (mmHg) | 119.2 ± 12.9 | 120.3 ± 12 | 0.507 |
| Diastolic blood pressure (mmHg) | 72.5 ± 7.7 | 75.3 ± 6.3 | 0.103 |
| Heart rate (bpm) | 79.2 ± 17.5 | 76.7 ± 17.3 | 0.291 |
| Body mass index (kg/m2) | 25.6 ± 3.9 | 25.1 ± 4.6 | 0.387 |
| Aspirin | 45 (%56.9) | 92 (%50.2) | 0.320 |
| Warfarin | 31 (%39.2) | 54 (%29.5) | 0.123 |
SEC: spontaneous echo contrast.
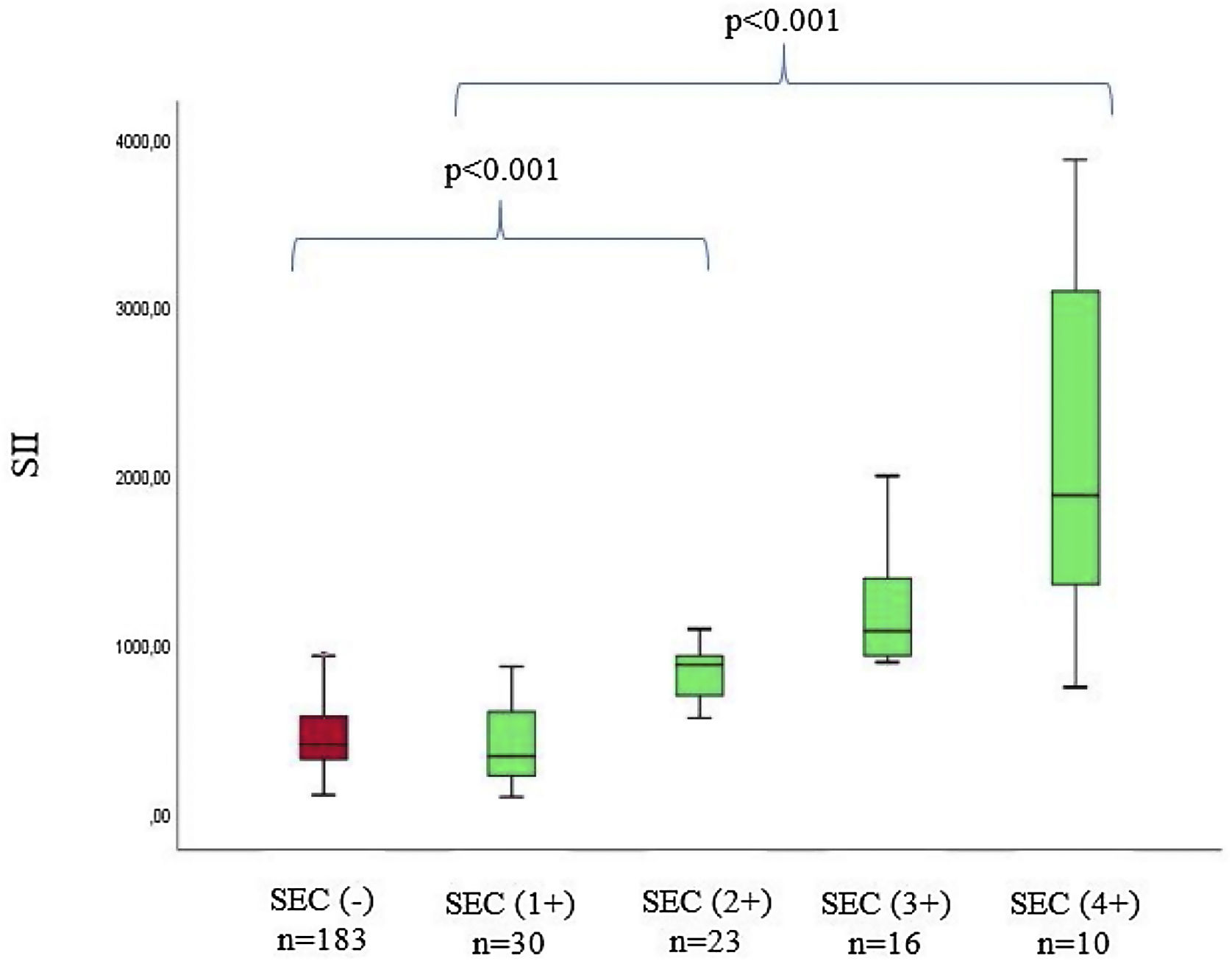
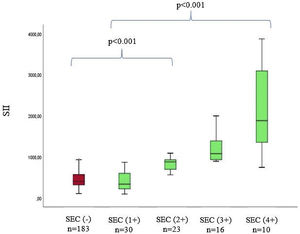
The baseline laboratory measurements of the study patients are listed in Table 2. Laboratory parameters were similar between groups, except for neutrophils, NLR, CRP, and SII levels. When SEC (+) patients and SEC (-) patients are compared, neutrophil levels (6.1 (3.6-7.4) vs. 3.1 (2.1-5.6), p<0.001), NLR levels (3.89 (1.84-6.47) vs. 1.82 (1.16-2.91), p<0.001), hs-CRP levels (5.21 ± 2.7 vs. 3.91 ± 2.5, p<0.001), and SII levels (869 (556-1112) vs. 378 (276-530), p<0.001) of SEC (+) patients were significantly higher. In addition, when we divided SEC (+) patients into four subgroups according to previously reported criteria, there was a positive correlation between SII levels and SEC grade (p<0.001) (Figure 1).
Laboratory findings of the study populations.
| SEC (+) | SEC (-) | p value | |
|---|---|---|---|
| Number of patients | (n=79) | (n=183) | |
| Creatinine (mg/dl) | 0.81 ± 0.1 | 0.71 ± 0.3 | 0.011 |
| AST (U/L) | 24.4 ± 10.1 | 25.4 ± 9.6 | 0.798 |
| ALT (U/L) | 27.7 ± 13.3 | 26.6 ± 12.1 | 0.667 |
| Total cholesterol (mg/dl) | 181.6 ± 43.5 | 173.4 ± 38.1 | 0.183 |
| HDL (mg/dl) | 34 ± 9.4 | 37.4 ± 8.1 | 0.088 |
| LDL (mg/dl) | 125.4 ± 41.7 | 115.6 ± 37.4 | 0.065 |
| Triglyceride (mg/dl) | 120.1 ± 62.7 | 125.9 ± 43.2 | 0.400 |
| Hemoglobin (mg/dL) | 14.3 ± 1.5 | 14.2 ± 1.6 | 0.610 |
| Platelets (103/μL) | 236.2 ± 61.1 | 227.4 ± 44 | 0.191 |
| WBC (103/μL) | 7.9 ± 2.7 | 7.2 ± 3.4 | 0.098 |
| Neutrophil (103/μL) | 6.1 (3.6-7.4) | 3.1 (2.1-5.6) | 0.001 |
| Lymphocyte (103/μL) | 1.71 ± 06 | 1.89 ± 08 | 0.087 |
| C reactive protein (CRP) | 5.21 ± 2.7 | 3.91 ± 2.5 | <0.001 |
| Neutrophil/Lymphocyte ratio (NLR) | 3.89 (1.84-6.47) | 1.82 (1.16-2.91) | <0.001 |
| Platelet/Lymphocyte ratio (PLR) | 168 (100-261) | 140 (105.5-203) | 0.275 |
| SII | 869 (556-1112) | 378 (276-530) | <0.001 |
HDL: high density lipoprotein cholesterol; LDL: low density lipoprotein cholesterol; NLR: neutrophil/lymphocyte ratio, PLR: platelets/lymphocyte ratio; SEC: spontaneous echo contrast; SII: systemic immune-inflammation index; WBC: white blood cell.
Echocardiographic parameters are shown in Table 3. The mean gradient of the mitral valve, right ventricle diameters, and LVEF did not differ between groups. In the SEC (+) group, the mitral valve area was significantly lower (1.0 ± 0.2 vs. 1.1 ± 0.2, p=0.009), while left atrial volume and systolic pulmonary artery pressure were significantly higher (76.9 ± 5.6 vs. 73.7 ± 9.3, p=0.005, 54.4 ± 6.3 vs. 51.6 ± 7.6, p=0.004) (Table 3).
Echocardiograph parameters of patients.
| SEC (+) | SEC (-) | p value | |
|---|---|---|---|
| (n=79) | (n=183) | ||
| LVEF (%) | 58.9 ± 7.4 | 60 ± 8.6 | 0.339 |
| LA volume (mL) | 76.9 ± 5.6 | 73.7 ± 9.3 | 0.005 |
| Mean gradient (mmHg) | 13 ± 1.5 | 12.7 ± 1.9 | 0.333 |
| Mitral valve area (cm2) | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.009 |
| PAP (mmHg) | 54.4 ± 6.3 | 51.6 ± 7.6 | 0.004 |
| RV diameter (cm) | 4.83 ± 0.29 | 4.67 ± 0.46 | 0.078 |
| Degree of SEC | |||
| 1(+) | 30 (%37.9) | ||
| 2(+) | 23 (%29.1) | ||
| 3(+) | 16 (%20.2) | ||
| 4(+) | 10 (%12.6) | ||
LA: left atrium; LVEF: left ventricular ejection fraction; PAP: pulmonary arterial pressure; RV: right ventricle; SEC: spontaneous echo contrast.
Relationships of multivariates with SEC were assessed using univariate and multivariate logistic regression analysis. In unadjusted logistic regression analysis, variables with unadjusted p<0.10, hs-CRP, SII, LA volume, RV diameter, mitral valve area, and PAP were identified as potential risk factors for SEC, and these variables were included in the multivariate logistic regression analysis (Table 4). To avoid multicollinearity, we omitted neutrophil, lymphocyte, and NLR in the regression models.
Univariate and multivariate predictors of spontaneous echo contrast in patients with mitral stenosis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | OR | 95% CI | p value | |
| SII | 1.001 | 1.001-1.002 | <0.001 | 1.001 | 1.000-1.001 | <0.001 |
| CRP | 1.193 | 1.074-1.325 | 0.001 | 1.145 | 1.027-1.277 | 0.014 |
| LA volume | 1.046 | 1.013-1.080 | 0.006 | 1.225 | 1.020-1.471 | 0.030 |
| Mean gradient | 1.072 | 0.931-1.235 | 0.334 | |||
| Mitral valve area | 0.191 | 0.054-0.672 | 0.010 | |||
| PAP | 1.056 | 1.017-1.096 | 0.004 | 1.043 | 1.000-1.087 | 0.047 |
| RV diameter | 1.746 | 0.935-3.260 | 0.081 | |||
CI: confidence interval; CRP: C-reactive protein; OR: odds ratio; PAP: pulmonary arterial pressure; RV: right ventricle; SII: systemic immune-inflammation index.
Multivariate logistic regression analysis demonstrated that high levels of SII were an independent risk factor for SEC in patients with MS (odds ratio (OR): 1.001, 95% confidence interval (CI): 1.000-1.001, p<0.001) together with CRP (OR: 1.344, 95% CI: 1.164-1.551, p=0.014). Moreover, LA volume (OR 1.225, 95% CI: 1.020-1.471, p=0.030) and PAP (OR: 1.043, 95% CI: 1.000-1.087, p=0.047) were also the independent risk factor for SEC in patients with MS (Table 4).
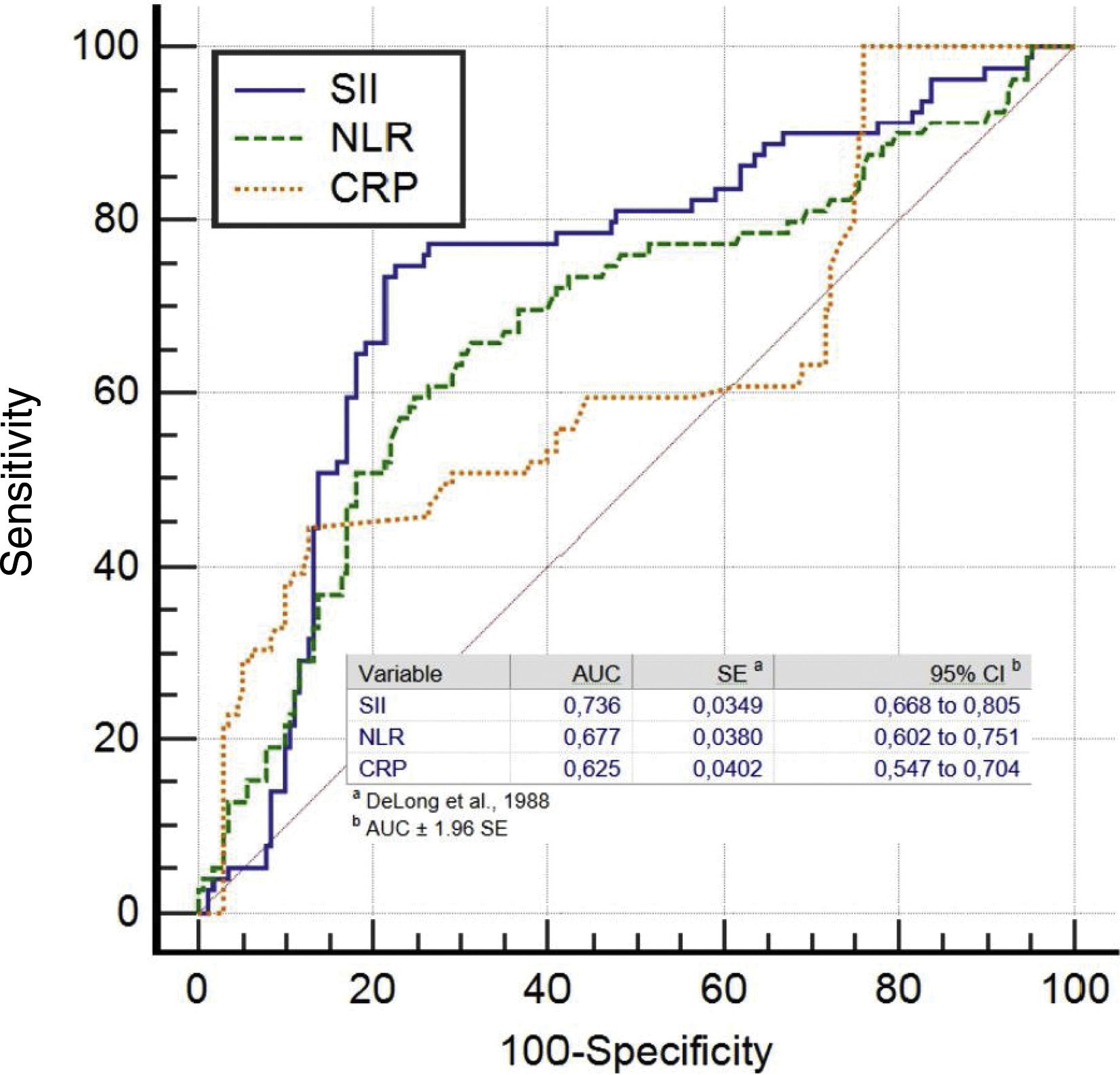
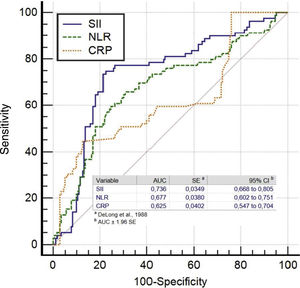
The ROC analysis showed that at a cutoff value of >547.6 for SII for predicting SEC with 74.6% sensitivity and 77.6% specificity (area under ROC curve=0.736 (95% CI: 0.668-0.805), p<0.001). For NLR, a cut-off value of 2.38 had predicting SEC with a sensitivity of 67.1% and specificity of 65%, (area under the curve (AUC)=0.677 (95% CI: 0.602–0.751, p<0.001). For CRP, a cut-off value of 3.14 had 60.8% sensitivity and 61.2% specificity in predicting SEC in patients with MS (area under ROC curve=0.625 (95% CI: 0.547-0.704), p<0.001) (Figure 2).
We found that SII had higher accuracy in predicting SEC than the NLR alone (SII vs. NLR: AUC: 0.736 vs. 0.677, z=5.872, p<0.0001). Also, the SII had similar discriminatory power in predicting SEC compared with the CRP levels (SII vs. CRP: AUC: 0.736 vs. 0.625, z=2.154, p=0.0031). However, the NLR and CRP had similar accuracy for predicting SEC (NLR vs. CRP: AUC: 0.677 vs. 0.625, z=1.044, p=0.2963).
DiscussionTo the best of our knowledge, this study is the first to show that there is an independent relationship between SII level and SEC in patients with mitral stenosis. ROC analysis revealed that the SII level in blood samples taken during admission predicted SEC with 74.6% sensitivity and 77.6% specificity for the cutoff value of 547.6. We also found that in patients with MS complicated by SEC, SII levels correlated with the degree of SEC. In addition to the increase in SII level, we showed that SEC risk was higher in patients with elevated CRP levels, increased LA volume, and higher PAP.
Mitral stenosis continues to be a significant health problem in developing countries. MS is essentially a cardiac problem that causes complaints due to the deterioration of valve function in the patient. However, non-cardiac systemic effects of MS also cause significant health problems, especially systemic thromboembolism and stroke. The frequent occurrence of AF in patients with MS may be an explanation for this situation. However, studies have shown that, regardless of AF, patients with MS have an increased tendency for thrombosis and embolic events are more common than those without MS.15 Studies showing the relationship between the dense amount of SEC in the LA and thrombus formation in stroke and LAA indicate that SEC is one of the reasons for this clinical result.16 However, the mechanisms behind the prothrombotic state are not fully understood. Therefore, it is essential to predict the pathophysiology and prothrombotic state that lead to SEC formation. In previous studies, several mechanisms thought to play a role in SEC physiopathology were mentioned. Blood stasis has been shown to play an essential role in the formation of SEC, especially in atrial fibrillation, mitral stenosis, or enlarged heart cavities due to the higher frequency of SEC.15 However, it has been shown in many studies that blood content has a vital role in the pathogenesis of SEC.17,18 Sigel et al. claimed that the most apparent cause of SEC is erythrocyte aggregation.18 Black et al. suggested that in addition to erythrocyte aggregation, intermittent platelet aggregation may contribute to the relationship between SEC and thromboembolism.7 Erbel et al. showed an increase in platelet aggregation in patients with SEC and that there is a regression in SEC with drugs that disrupt platelet aggregation.19 Recent studies have shown that inflammation and increase in various inflammatory markers are closely related to SEC formation and a prothrombotic state.7–9
Abu-Mahfouz et al., in a prospective study in patients with low thrombotic risk, showed a relationship between acute phase reactant and inflammation marker CRP and intracardiac thrombus and SEC formation.20 Ederhy et al. found that a CRP value of 3.4 mg/L in patients with AF has a high negative predictive value to exclude the presence of LA or LAA thrombus.21
Kaya et al., in their study of 62 MS patients, showed that patients with SEC had higher CRP, neutrophil, and NLR levels compared to patients without SEC. They also found that the ratio of CRP and NLR in MS patients was independently associated with SEC.8 A recent study by Sahin et al. showed that platelet-to-lymphocyte ratio (PLR) 123 value has 71% sensitivity and 52% specificity in predicting SEC in patients with mitral stenosis.9
In this study, the elevation of CRP and increased NLR level were associated with SEC formation in accordance with the studies mentioned earlier. However, we could not find a relationship between the PLR level and SEC. This result we found was incompatible with the observations of Sahin et al. The most striking finding of our study is that the increase in SII level, which has been recently introduced as a new inflammation parameter, stands out as a powerful independent predictor of SEC in patients with MS. In the SEC subgroup, SII levels also correlated with SEC grade. Although both NLR and CRP levels were statistically and independently associated with SEC in patients with mitral stenosis, they did not gradually increase with the intensity of SEC, as in SII.
The idea has emerged that SII, which is a recently introduced inflammatory index consisting of the combination of three inflammatory cell types (neutrophils, lymphocytes, and platelets) can more comprehensively depict and describe the immune and inflammatory condition in patients compared to inflammatory predictors based on one (such as neutrophils and lymphocytes) or two components (such as NLR and PLR). Recent studies have shown that high SII levels are superior to NLR and PLR in predicting the risk of adverse clinical outcomes in various diseases, thus supporting this idea.10,22,23 Having initially been proven to be vital in relation to prognosis in some types of cancer, the use of SII in cardiovascular diseases has become even more popular after Seo et al. showed this marker's predictive value in patients with chronic heart failure for the first time.24 Later, Huang et al. found that in STEMI patients treated with percutaneous coronary intervention, increased SII levels were associated with short- and long-term poor clinical outcomes.10 Kelesoglu et al., on the other hand, argued that high SII levels could be a promising indicator for contrast-induced nephropathy in patients with non-ST segment elevation myocardial infarction.23 In our study, on the other hand, we found that among the inflammatory markers examined in MS patients treated with PMBV, the strongest and independent marker associated with SEC development was SII. Moreover, we found that the optimum cutoff point for SII was 547.6, which predicted the risk of SEC development with 74.6% sensitivity and 77.6% specificity. In the SEC subgroup, SII levels also correlated with SEC grade.
We believe that this relationship between SII and SEC is due to certain mechanisms. In previous studies, hypercoagulation status was reported in patients with mitral stenosis, whatever the rhythm.25,26 As it is widely known, platelets play a major role in the hemostatic system and a critical role in the activation of intrinsic pathway factors.27 In addition to the coagulation system, platelets may play an important role in developing thromboembolic events in patients with mitral stenosis. Studies have shown that platelet activity increases in patients with left atrial SEC.7,19,20 Consequently, both inflammation and aggregation pathways play critical roles in left atrial SEC formation, consistent with previous evidence. All these findings suggest that SII, a biomarker that combines information from both inflammation and aggregation pathways, may play a key role in forming LA SEC in MS patients. Based on our study, we speculate that SEC (+) patients with MS with high SII levels might gain some advantage from intensive antiplatelet therapy and decrease their risk of thromboembolic events.
Study limitationsThe most important limiting factor was the relatively small number of patients in our study. The study was a single-center retrospective study, and that the selected population did not represent the entire cohort. Apart from this, the main study limitation was that SII levels were calculated only from blood samples taken immediately after TEE at the time of hospitalization. This was not re-evaluated during hospital follow-up. More research is needed to determine the inflammatory status in patients better and determine when to take blood sampling. In addition, parameters such as left atrial appendage (LAA) emptying velocities, which show LAA functions, could not be evaluated in this study.
One of the most critical features of the current study is comparing multiple inflammatory markers (NLR, PLR, CRP), which were previously associated with SEC formation in patients with MS. Recent study results show that SII, which shows the inflammatory state as a practical, simple, easily measurable, and inexpensive indicator, is the strongest inflammatory marker that predicts the risk of SEC formation in patients with MS.
Author contributionsStudy concept and design: SK, DE, MTI, Literature research, and clinical advice: SK, IZ, AD, AO, Acquisition, analysis, or interpretation of data: SK, DE, NK, RO, Drafting of the manuscript: SK, DE, MTI, Critical revision of the manuscript for important intellectual content: DE, SK, NK, Statistical analyses: DE, Study supervision: MTI, NK. All authors read and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.