The association of low-density lipoprotein cholesterol (LDL-C) levels and prognosis in patients with heart failure (HF) remains uncertain. This study aimed to evaluate the prognostic significance of LDL-C in patients admitted for acutely decompensated HF and establish a safety cut-off value in this population.
MethodsThis retrospective, observational study included 167 consecutive patients admitted for acute HF. LDL-C levels were measured on hospital admission, and patients were categorized according to their estimated cardiovascular (CV) risk. The primary endpoint was all-cause mortality at one-year, while secondary endpoints included HF hospitalizations, major thrombotic events, and net clinical benefit.
ResultsDuring the follow-up period, 14.4% of patients died. Higher LDL-C levels were independently associated with improved survival, with a 4-fold increase in survival probability for each 1 mg/dL increase in serum LDL-C. The minimum LDL-C value not associated with increased mortality risk was 88 mg/dL. Patients with LDL-C below this cut-off had a significantly higher risk of mortality and a tendency for higher HF hospitalization risk. The net clinical benefit endpoint was also influenced by LDL-C levels, with LDL-C below 88 mg/dL associated with an increased risk of events.
ConclusionIn patients admitted for acutely decompensated HF, higher LDL-C levels were associated with reduced risk of mortality. An LDL-C value below 88 mg/dL was associated with increased mortality, suggesting the need for a more liberal LDL-C target in this specific patient population. These findings highlight the importance of considering LDL-C levels in the management and risk assessment of patients with HF.
A associação entre os níveis de colesterol de lipoproteína de baixa densidade (LDL-C) e o prognóstico de doentes com insuficiência cardíaca (IC) permanece incerto. Este estudo teve como objetivo avaliar o valor prognóstico dos níveis de LDL-C em doentes admitidos por IC aguda e estabelecer um cut-off de segurança para esta população.
MétodosEste estudo retrospetivo e observacional incluiu 167 doentes admitidos por IC aguda. Os níveis de LDL-C foram avaliados no momento de admissão hospitalar e os doentes foram classificados de acordo com o seu risco cardiovascular. O outcome primário do estudo foi mortalidade de qualquer causa a um ano e os outcomes secundários incluíram hospitalizações por IC, eventos trombóticos major e benefício clínico global.
ResultadosDurante o período de seguimento, 14,4% dos doentes faleceram. Níveis de LDL-C mais elevados associaram-se de forma independentemente a aumento de sobrevida, com um de 4 vezes na probabilidade de sobrevida por cada aumento de 1 mg/dL no LDL-C sérico. O valor mínimo de LDL-C que não se associou a aumento de mortalidade foi 88 mg/dL. Nos doentes com LDL-C abaixo deste valor observou-se um aumento significativo do risco de mortalidade, bem como uma tendência para um aumento do risco de hospitalização por IC. O benefício clínico global também foi influenciado pelos níveis de LDL-C, com níveis abaixo de 88 mg/dL associados a um aumento no risco de eventos.
ConclusãoEm doentes admitidos por IC aguda descompensada, níveis mais elevados de LDL-C associaram-se a uma redução do risco de mortalidade. Este estudo identificou como cut-off de segurança o valor de LDL-C de 88 mg/dL, sugerindo a necessidade de um alvo de LDL-C mais liberal nesta população específica. Estes resultados reforçam a importância de considerar os níveis de LDL-C na gestão e avaliação de riscos de doentes com IC.
Heart failure (HF) is a leading cause of morbidity and mortality worldwide. In developed countries its prevalence is estimated at 1–2% and its incidence is rising.1 Currently, the main causes of HF are hypertension and coronary artery disease. As the most impactful risk factor for the development and progression of atherosclerosis, dyslipidemia,2 seems to have a role in HF pathophysiology in some patients.
HMG-CoA reductase inhibitors reduce cholesterol levels and subsequently diminish the risk of major vascular events and associated morbidity and mortality,3 as well the risk HF development.4,5 Two major randomized trials evaluated the impact of rosuvastatin in chronic HF patients in NYHA class II–IV and failed to provide an evident beneficial effect. Interestingly, only patients with HF due to ischemic heart disease (IHD) were included in the CORONA trial and IHD represented 40% of the GISSI-HF trial population, revealing that these results may be independent of HF etiology.6,7 Based on current recommendations, routine administration of statins in HF patients is not recommended, unless there is another indication for their use.1,7
Recent trials IMPROVE-IT, FOURIER, ODYSSEY and metanalyses including over 200000 patients reported a significant reduction in cardiovascular (CV) events with low-density lipoprotein cholesterol (LDL-C) levels below the previous established targets.8–10 Based on these results tighter LDL-C targets were recommended in the 2019 European Society of Cardiology (ESC) guidelines on Dyslipidemia and Cardiovascular Prevention.11,12 Importantly, in these trials only a minority of patients had co-existing HF (5% in IMPROVE-IT and 15% in ODISSEY).8,10
Previous studies reported that low cholesterol levels were independently associated with adverse outcomes and mortality in patients with chronic HF.13–15 A similar association was also observed in patients admitted for acutely decompensated HF, in whom total cholesterol was inversely related to, length of stay, symptoms at discharge, in-hospital mortality16 and also post-discharge mortality.17
Considering the mentioned correlation between low cholesterol levels and chronic HF prognosis it is unclear whether currently recommended CV risk-based LDL-C targets for general population should be applied to patients with established HF.
ObjectivesThe aim of this study was to evaluate the association between LDL-C levels and prognosis in patients admitted for acutely decompensated HF and to establish the lowest LDL-C value associated with worse prognosis in this specific group of patients.
MethodsDesign and populationThis was a single-center, observational, retrospective study of consecutive patients admitted for acute HF from January 2016 to December 2018 with a lipid panel performed on hospital admission. Patients admitted due to acute myocardial infarction were excluded from analysis.
Heart failure diagnosis and treatment were performed according to the recommendations present in the ESC HF guidelines.1
LDL-C levels were estimated using the Friedwald formula.18 Demographic, clinical, laboratory, echocardiographic and therapeutic data regarding index hospitalization were collected. All patients were categorized according to the estimated CV risk as suggested by the ESC Dyslipidemia Guidelines.11
The study was approved by the local ethics committee and by the National Data Protection Authority. Patient confidentiality was ensured through anonymization of the collected data. All study procedures were carried out in accordance with the ethical principles expressed in the 2013 revision of the Declaration of Helsinki.19
Study outcomesThe primary endpoint was all-cause mortality at one-year follow-up. The secondary endpoints were (1) HF hospitalizations, (2) major thrombotic events (stroke or acute coronary syndrome) and (3) net clinical benefit defined by the composite of all-cause mortality, HF hospitalizations and thrombotic events.
Statistical analysisContinuous variables were described using mean and standard deviation for normally distributed data or median and interquartile range (IQR) for non-normally distributed data. Relationships between continuous variables were established using the Student's T or Mann–Whitney U tests, respectively. Categorical variables were expressed as counts or percentages and were compared using the Chi-square test.
Impact of LDL-C on the study endpoints was evaluated using Cox-regression analysis adjusted for age, gender, left ventricle ejection fraction (LVEF), NYHA functional class and HF etiology and for other relevant metabolic factors – body mass index (BMI) and glycated hemoglobin (HbA1c).
The lowest LDL-C value associated with mortality was estimated with receiver operator curve (ROC) and Youden's index and its association with prognosis was evaluated using Kaplan–Meier analysis.
All statistical analysis were performed with Statistical Package for Social Sciences (SPSS) version 26 (ISPSS®).
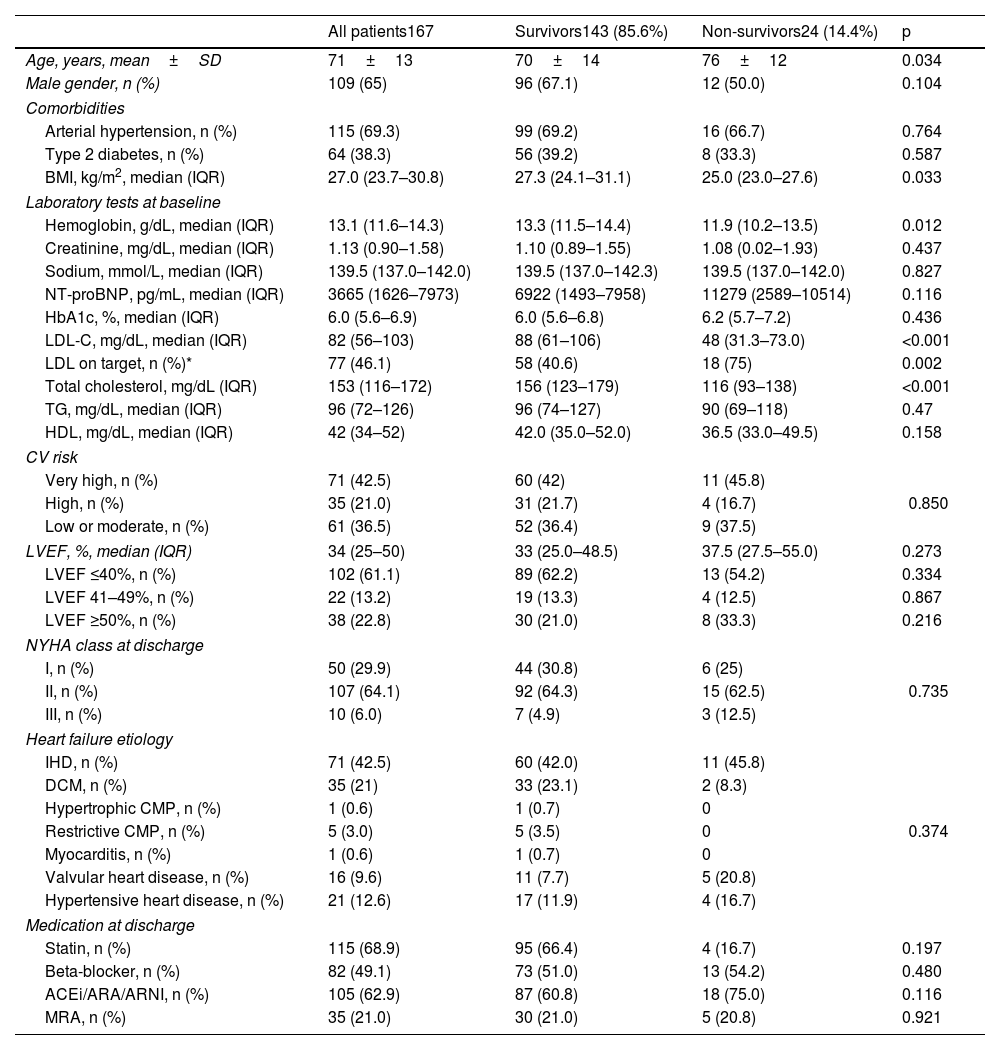
ResultsPopulation characterizationFrom a total of 224 patients admitted due to acute HF, 167 fulfilled the inclusion criteria. Baseline demographic and clinical data are presented in Table 1. The population presented a mean age of 71±13 years and 65% were male. The main causes of HF were IHD in 71 (42.5%) patients and dilated cardiomyopathy (DCM) in 35 (21%). A predominance of HF with reduced ejection fraction was observed (61.1%) and the overall median LVEF was 34% (IQR 25–50). Stratification according to CV risk suggested by the ESC Dyslipidaemia and Cardiovascular Prevention Guidelines was the following: 42.5% patients had very-high risk, 21% had high risk and 36.5% had low to moderate risk. At discharge most patients (64.1%) were in class NYHA II.
Population characteristics at baseline.
| All patients167 | Survivors143 (85.6%) | Non-survivors24 (14.4%) | p | |
|---|---|---|---|---|
| Age, years, mean±SD | 71±13 | 70±14 | 76±12 | 0.034 |
| Male gender, n (%) | 109 (65) | 96 (67.1) | 12 (50.0) | 0.104 |
| Comorbidities | ||||
| Arterial hypertension, n (%) | 115 (69.3) | 99 (69.2) | 16 (66.7) | 0.764 |
| Type 2 diabetes, n (%) | 64 (38.3) | 56 (39.2) | 8 (33.3) | 0.587 |
| BMI, kg/m2, median (IQR) | 27.0 (23.7–30.8) | 27.3 (24.1–31.1) | 25.0 (23.0–27.6) | 0.033 |
| Laboratory tests at baseline | ||||
| Hemoglobin, g/dL, median (IQR) | 13.1 (11.6–14.3) | 13.3 (11.5–14.4) | 11.9 (10.2–13.5) | 0.012 |
| Creatinine, mg/dL, median (IQR) | 1.13 (0.90–1.58) | 1.10 (0.89–1.55) | 1.08 (0.02–1.93) | 0.437 |
| Sodium, mmol/L, median (IQR) | 139.5 (137.0–142.0) | 139.5 (137.0–142.3) | 139.5 (137.0–142.0) | 0.827 |
| NT-proBNP, pg/mL, median (IQR) | 3665 (1626–7973) | 6922 (1493–7958) | 11279 (2589–10514) | 0.116 |
| HbA1c, %, median (IQR) | 6.0 (5.6–6.9) | 6.0 (5.6–6.8) | 6.2 (5.7–7.2) | 0.436 |
| LDL-C, mg/dL, median (IQR) | 82 (56–103) | 88 (61–106) | 48 (31.3–73.0) | <0.001 |
| LDL on target, n (%)* | 77 (46.1) | 58 (40.6) | 18 (75) | 0.002 |
| Total cholesterol, mg/dL (IQR) | 153 (116–172) | 156 (123–179) | 116 (93–138) | <0.001 |
| TG, mg/dL, median (IQR) | 96 (72–126) | 96 (74–127) | 90 (69–118) | 0.47 |
| HDL, mg/dL, median (IQR) | 42 (34–52) | 42.0 (35.0–52.0) | 36.5 (33.0–49.5) | 0.158 |
| CV risk | ||||
| Very high, n (%) | 71 (42.5) | 60 (42) | 11 (45.8) | 0.850 |
| High, n (%) | 35 (21.0) | 31 (21.7) | 4 (16.7) | |
| Low or moderate, n (%) | 61 (36.5) | 52 (36.4) | 9 (37.5) | |
| LVEF, %, median (IQR) | 34 (25–50) | 33 (25.0–48.5) | 37.5 (27.5–55.0) | 0.273 |
| LVEF ≤40%, n (%) | 102 (61.1) | 89 (62.2) | 13 (54.2) | 0.334 |
| LVEF 41–49%, n (%) | 22 (13.2) | 19 (13.3) | 4 (12.5) | 0.867 |
| LVEF ≥50%, n (%) | 38 (22.8) | 30 (21.0) | 8 (33.3) | 0.216 |
| NYHA class at discharge | ||||
| I, n (%) | 50 (29.9) | 44 (30.8) | 6 (25) | 0.735 |
| II, n (%) | 107 (64.1) | 92 (64.3) | 15 (62.5) | |
| III, n (%) | 10 (6.0) | 7 (4.9) | 3 (12.5) | |
| Heart failure etiology | ||||
| IHD, n (%) | 71 (42.5) | 60 (42.0) | 11 (45.8) | 0.374 |
| DCM, n (%) | 35 (21) | 33 (23.1) | 2 (8.3) | |
| Hypertrophic CMP, n (%) | 1 (0.6) | 1 (0.7) | 0 | |
| Restrictive CMP, n (%) | 5 (3.0) | 5 (3.5) | 0 | |
| Myocarditis, n (%) | 1 (0.6) | 1 (0.7) | 0 | |
| Valvular heart disease, n (%) | 16 (9.6) | 11 (7.7) | 5 (20.8) | |
| Hypertensive heart disease, n (%) | 21 (12.6) | 17 (11.9) | 4 (16.7) | |
| Medication at discharge | ||||
| Statin, n (%) | 115 (68.9) | 95 (66.4) | 4 (16.7) | 0.197 |
| Beta-blocker, n (%) | 82 (49.1) | 73 (51.0) | 13 (54.2) | 0.480 |
| ACEi/ARA/ARNI, n (%) | 105 (62.9) | 87 (60.8) | 18 (75.0) | 0.116 |
| MRA, n (%) | 35 (21.0) | 30 (21.0) | 5 (20.8) | 0.921 |
ACEi: angiotensin-converting enzyme inhibitor; ARA: angiotensin II receptor antagonists; ARNI: angiotensin receptor-neprilysin inhibitor; BMI: body mass index; CMP: cardiomyopathy; CV: cardiovascular; DCM: dilated cardiomyopathy; HbA1c: glycated hemoglobin; HDL: high-density cholesterol; IHD: ischemic heart disease; IQR: interquartile range; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; MRA: mineralocorticoids receptor antagonists; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association.
Discharge medication included statins in 69% of patients, beta-blockers in 49%, mineralocorticoid receptor antagonists in 21% and renin–angiotensin–aldosterone system inhibitors (angiotensin-converting-enzyme inhibitors – ACEi, angiotensin receptor antagonists – ARB or angiotensin receptor/neprilysin inhibitor – ARNI) in 62.9%.
Patients presented a median LDL-C level of 82 mg/dL (56–103) and 45.5% of the patients had an LDL-C level below the target proposed for their estimated CV risk in the ESC Dyslipidaemia and Cardiovascular Prevention Guidelines.
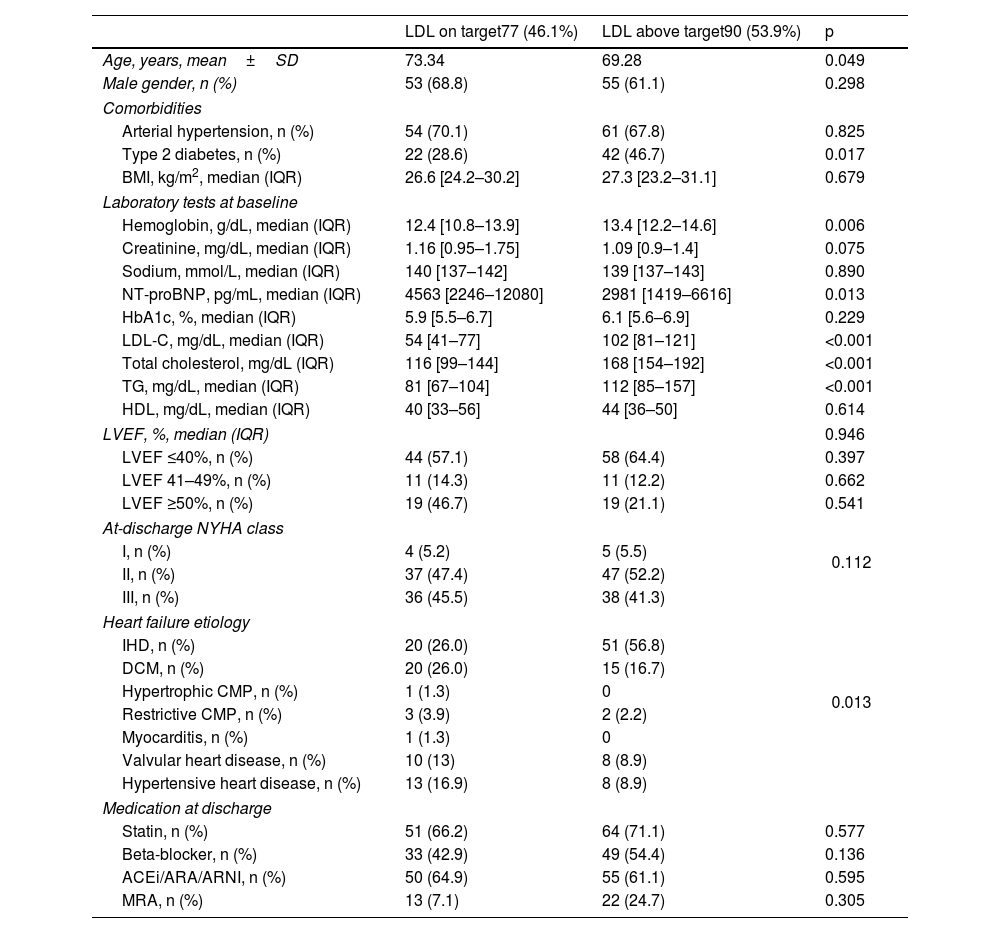
As expected, patients with LDL-C below their recommend target presented lower LDL-C (p<0.001), total cholesterol (p<0.001) and triglycerides (p<0.001) levels. These patients were older (p=0.005) and had less frequently diabetes (p=0.017) and IHD (p=0.013). They also presented lower hemoglobin (p=0.006) and higher NT-proBNP (p=0.013) levels on admission. No relation was observed between serum LDL-C level and LVEF (p=0.820) or NYHA functional class at discharge (p=0.735). Additionally, LDL-C level was not related to BMI (p=0.679) or HbA1c (p=0.229). Unexpectedly, statin prescription rates were similar between patients with LDL-C below and above the target (66.2% vs 71.1%, p=0.577) (Table 2).
Population stratified according to the ESC guidelines LDL-C level target.
| LDL on target77 (46.1%) | LDL above target90 (53.9%) | p | |
|---|---|---|---|
| Age, years, mean±SD | 73.34 | 69.28 | 0.049 |
| Male gender, n (%) | 53 (68.8) | 55 (61.1) | 0.298 |
| Comorbidities | |||
| Arterial hypertension, n (%) | 54 (70.1) | 61 (67.8) | 0.825 |
| Type 2 diabetes, n (%) | 22 (28.6) | 42 (46.7) | 0.017 |
| BMI, kg/m2, median (IQR) | 26.6 [24.2–30.2] | 27.3 [23.2–31.1] | 0.679 |
| Laboratory tests at baseline | |||
| Hemoglobin, g/dL, median (IQR) | 12.4 [10.8–13.9] | 13.4 [12.2–14.6] | 0.006 |
| Creatinine, mg/dL, median (IQR) | 1.16 [0.95–1.75] | 1.09 [0.9–1.4] | 0.075 |
| Sodium, mmol/L, median (IQR) | 140 [137–142] | 139 [137–143] | 0.890 |
| NT-proBNP, pg/mL, median (IQR) | 4563 [2246–12080] | 2981 [1419–6616] | 0.013 |
| HbA1c, %, median (IQR) | 5.9 [5.5–6.7] | 6.1 [5.6–6.9] | 0.229 |
| LDL-C, mg/dL, median (IQR) | 54 [41–77] | 102 [81–121] | <0.001 |
| Total cholesterol, mg/dL (IQR) | 116 [99–144] | 168 [154–192] | <0.001 |
| TG, mg/dL, median (IQR) | 81 [67–104] | 112 [85–157] | <0.001 |
| HDL, mg/dL, median (IQR) | 40 [33–56] | 44 [36–50] | 0.614 |
| LVEF, %, median (IQR) | 0.946 | ||
| LVEF ≤40%, n (%) | 44 (57.1) | 58 (64.4) | 0.397 |
| LVEF 41–49%, n (%) | 11 (14.3) | 11 (12.2) | 0.662 |
| LVEF ≥50%, n (%) | 19 (46.7) | 19 (21.1) | 0.541 |
| At-discharge NYHA class | 0.112 | ||
| I, n (%) | 4 (5.2) | 5 (5.5) | |
| II, n (%) | 37 (47.4) | 47 (52.2) | |
| III, n (%) | 36 (45.5) | 38 (41.3) | |
| Heart failure etiology | 0.013 | ||
| IHD, n (%) | 20 (26.0) | 51 (56.8) | |
| DCM, n (%) | 20 (26.0) | 15 (16.7) | |
| Hypertrophic CMP, n (%) | 1 (1.3) | 0 | |
| Restrictive CMP, n (%) | 3 (3.9) | 2 (2.2) | |
| Myocarditis, n (%) | 1 (1.3) | 0 | |
| Valvular heart disease, n (%) | 10 (13) | 8 (8.9) | |
| Hypertensive heart disease, n (%) | 13 (16.9) | 8 (8.9) | |
| Medication at discharge | |||
| Statin, n (%) | 51 (66.2) | 64 (71.1) | 0.577 |
| Beta-blocker, n (%) | 33 (42.9) | 49 (54.4) | 0.136 |
| ACEi/ARA/ARNI, n (%) | 50 (64.9) | 55 (61.1) | 0.595 |
| MRA, n (%) | 13 (7.1) | 22 (24.7) | 0.305 |
Abbreviations as in Table 1.
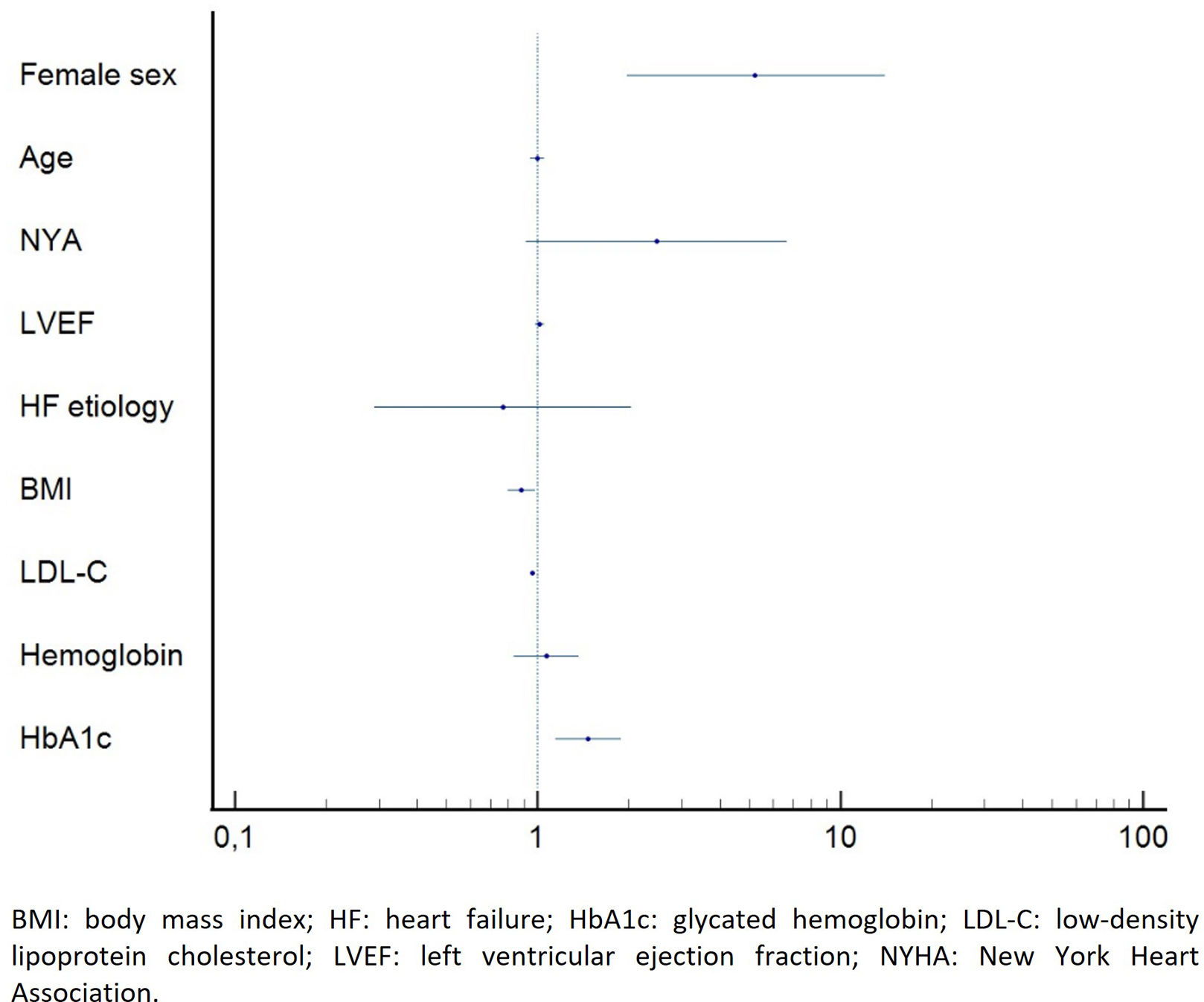
During follow-up 24 (14.4%) patients died. Serum LDL-C, hemoglobin and BMI and female gender were significantly associated with mortality (Figure 1). After adjustment for clinically relevant factors on multivariate analysis, higher LDL-C and BMI were independent protective factors for mortality, with a 4-fold increase in survival probability for each 1 mg/dL increase in serum LDL-C (HR 0.953 [0.915–0.993]; p<0.001) and a 12-fold increase in survival probability for each 1 kg/m2 increase in BMI (HR 0.884 [0.796–0.982]; p=0.022). On the other hand, female gender and HbA1c were independent predictors of mortality (HR 5.272 [1.983–14.015], p<0.001; HR 1.469 [1.142–1.889], p=0.003; respectively) (Figure 1). In comparison to men, women were significantly older (76 years [67–84] vs. 70 years [64–79], p=0.015), presented lower hemoglobin values (12.3 mg/dL [11.0–13.5] vs. 13.5 mg/dL [11.7–14.8] p=0.001) and a slightly higher median LVEF (37.5% [25.3–55.0] vs. 32.5% [25.0–46.0], p=0.049).
Even when considering only the patients at high and very-high CV risk, higher LDL-C level was still a protective factor for one-year mortality on multivariate analysis (HR 0.957 [0.931–0.983], p=0.001). In this subset of patients, HbA1c was the only predictor of mortality (HR 1.725 [1.265–2.343], p=0.001).
When considering the individual CV risk, patients with an LDL-C level below the recommended target presented an increased risk of mortality (HR 3.761 [1.493–9.478], p=0.005). The use of statins had no impact on survival (p=0.714) independently of the estimated CV risk.
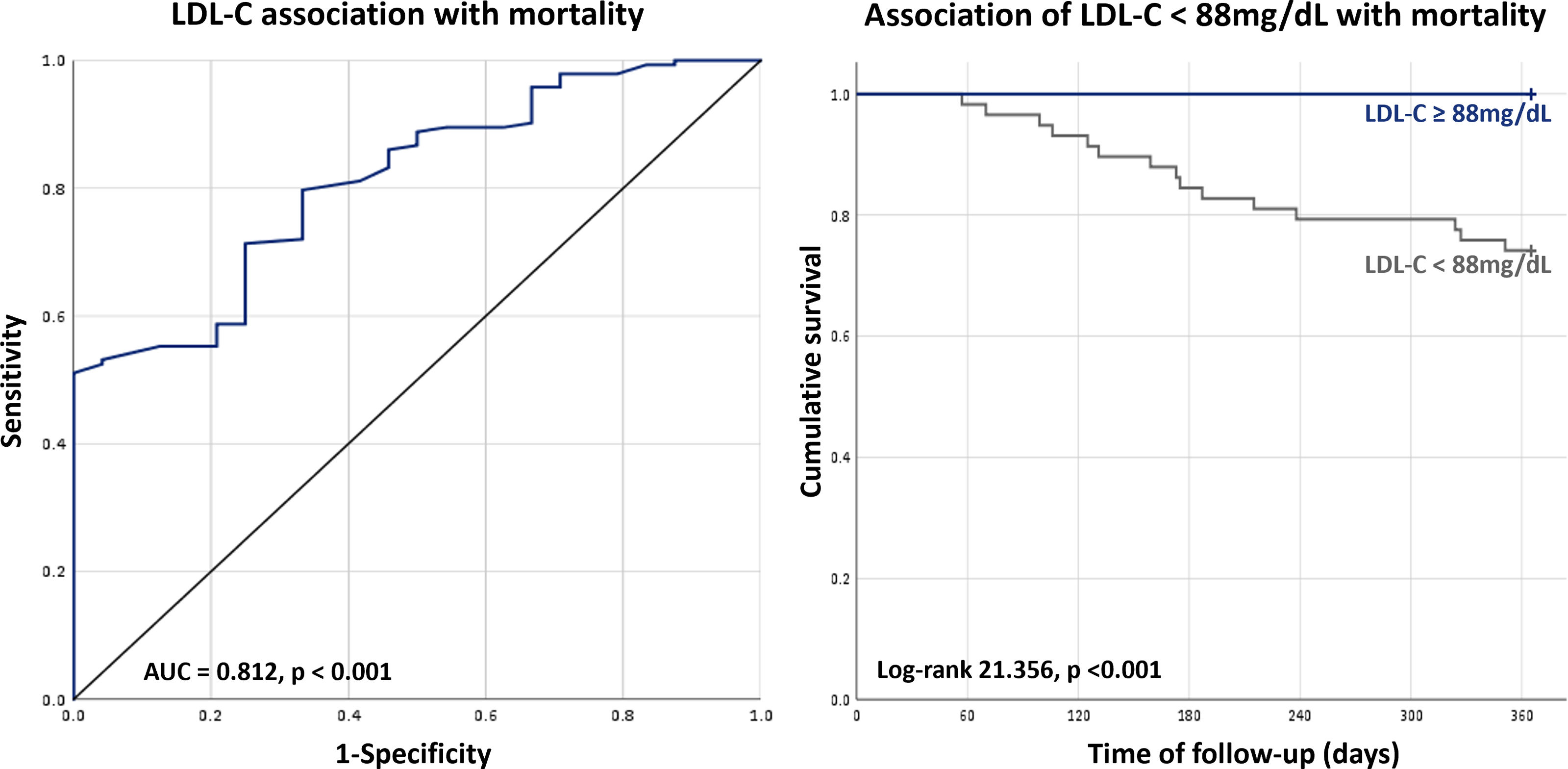
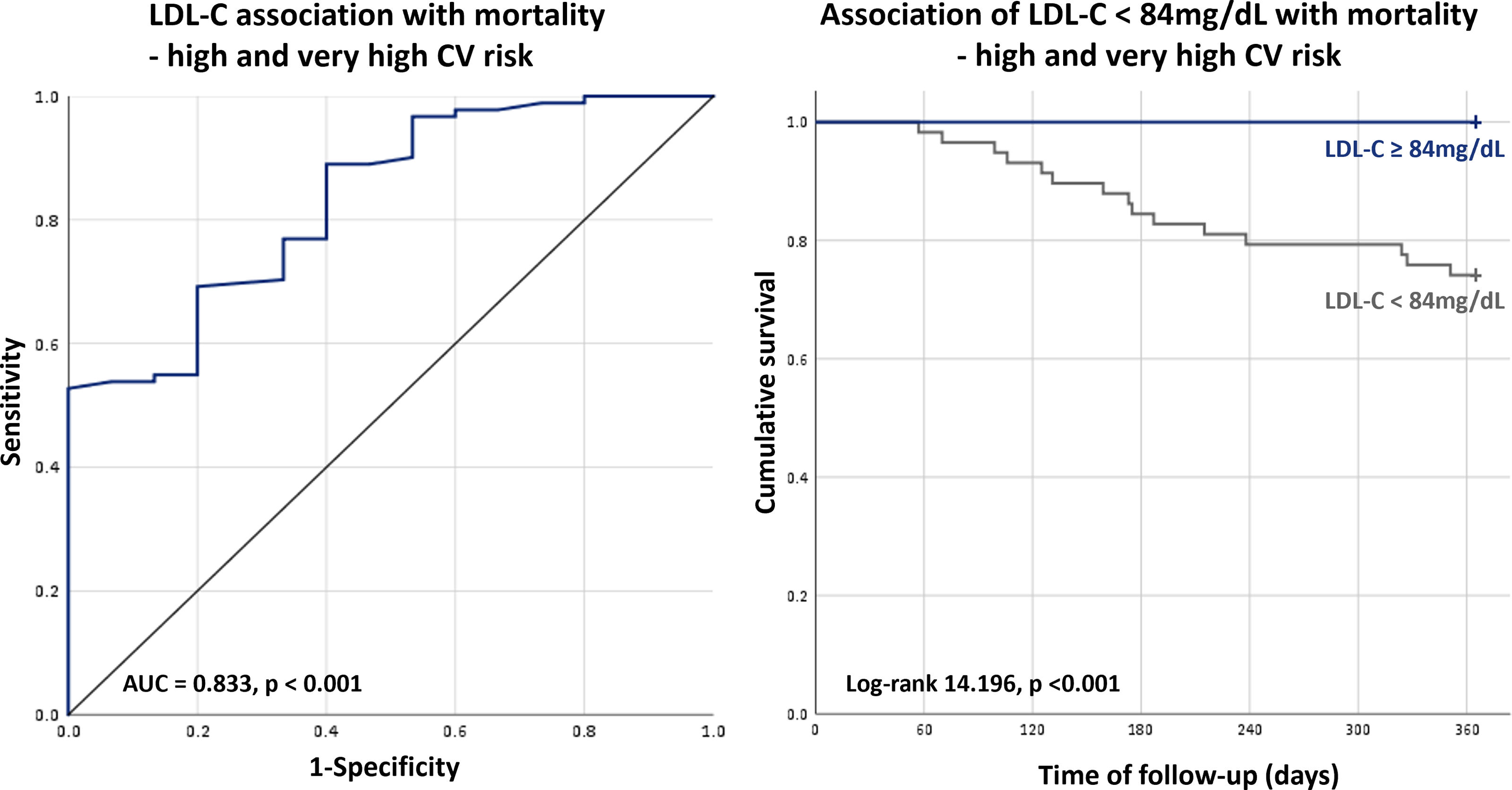
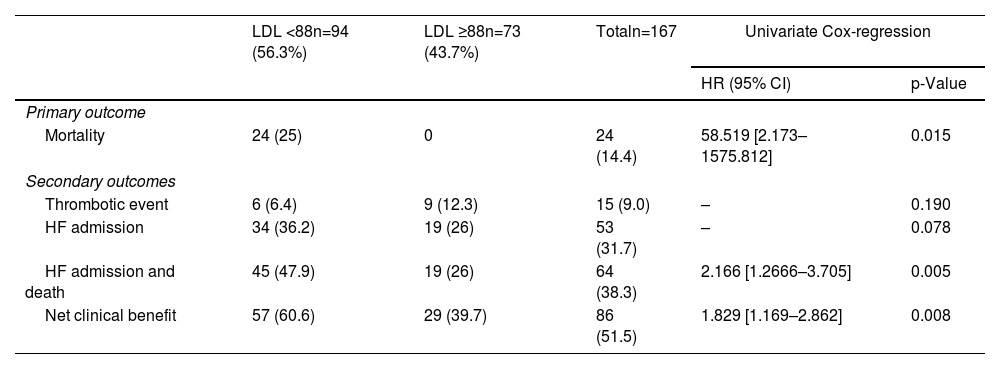
In the overall population, the minimum value of LDL-C not associated with increased risk of one-year mortality was 88 mg/dL (AUC 0.812, p<0.001; sensitivity: 51.4%; specificity: 100%), whereas in patients at high and very-high CV risk a cut-off of 84 mg/dL was found (AUC 0.833, p<0.001; sensitivity: 52.7%; specificity: 100%). In the overall population, an LDL-C level below 88 mg/dL was associated with a markedly increased risk of mortality (HR 58.51 [2.173–1575], p=0.015) (Table 3) and the same was evident for the high and very-high CV risk population using the cut-off of 84 mg/dL (HR 22.71 [3.066–168.3], p=0.02) (Figures 2 and 3).
Event rate according to the LDL-C threshold of 88 mg/dL.
| LDL <88n=94 (56.3%) | LDL ≥88n=73 (43.7%) | Totaln=167 | Univariate Cox-regression | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | ||||
| Primary outcome | |||||
| Mortality | 24 (25) | 0 | 24 (14.4) | 58.519 [2.173–1575.812] | 0.015 |
| Secondary outcomes | |||||
| Thrombotic event | 6 (6.4) | 9 (12.3) | 15 (9.0) | – | 0.190 |
| HF admission | 34 (36.2) | 19 (26) | 53 (31.7) | – | 0.078 |
| HF admission and death | 45 (47.9) | 19 (26) | 64 (38.3) | 2.166 [1.2666–3.705] | 0.005 |
| Net clinical benefit | 57 (60.6) | 29 (39.7) | 86 (51.5) | 1.829 [1.169–2.862] | 0.008 |
HF: heart failure; LDL-C: low-density lipoprotein cholesterol.
During follow-up, 53 (31.7%) patients were hospitalized due to HF and 72 (43.1%) due to any cause. Fifteen (9.0%) patients had a thrombotic event, of whom 3 (1.8%) had a stroke and 12 (5.3%) an acute coronary syndrome.
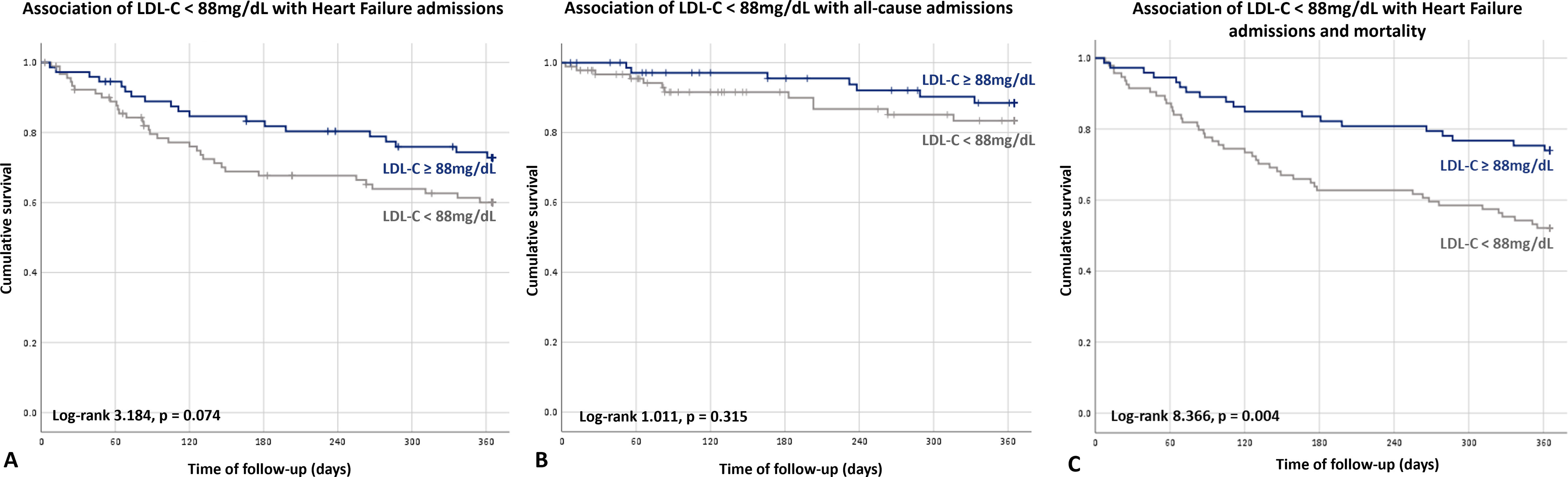
No statistically significant predictive factors for HF or all-cause admission were found. Nevertheless, although it was more evident for HF admissions, there was a trend showing an increase in both HF and all-cause admissions in patients with LDL-C level below the estimated value associated with mortality 88mg/dL (26.0% vs 37.0%, p=0.074; 35.6% vs 48.9%, p=0.315; respectively) (Figure 4).
Considering the composite endpoint of mortality and HF admission, serum LDL-C was the only predictive factor found on multivariate analysis (HR 0.991 [0.983–1.000], p=0.036). Also, an LDL-C level below 88 mg/dL was associated with a 2-fold increase in the risk of mortality or HF hospitalization (HR 1972 [1.235–3155], p=0.004) (Figure 4).
However, on multivariate analysis, higher LDL-C levels were also associated with an increased risk of thrombotic events (HR 1.012 [1.001–1.025], p=0.047). The main predictor for thrombotic events was having IHD (HR 19.299 [2.411–152.881]; p=0.005). An LDL-C level below 88 mg/dL in the overall population and below 84 mg/dL in the high and very-high CV risk population was not associated with an increased risk of thrombotic events.
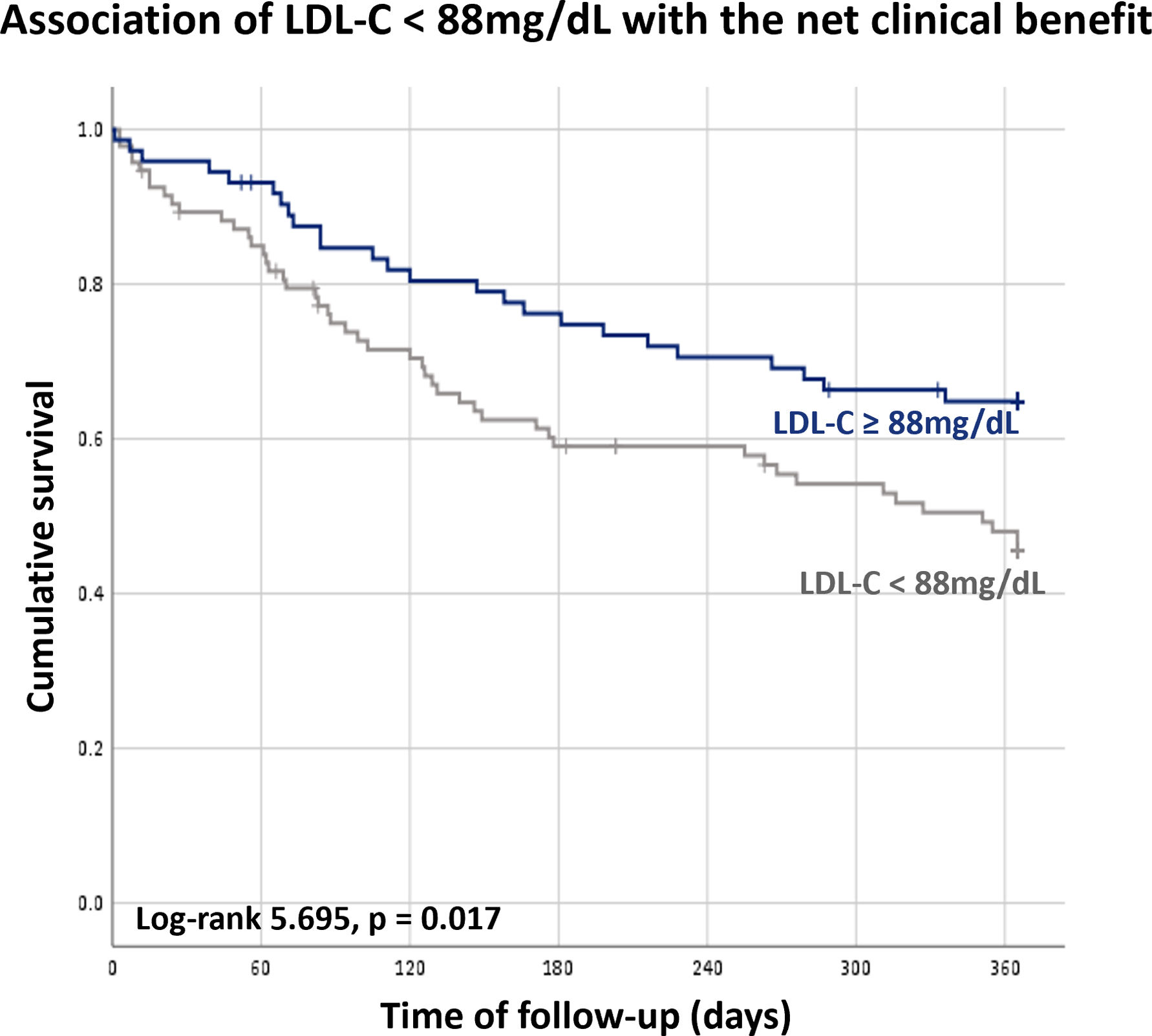
Although the LDL-C level was not associated with higher probability of the net clinical benefit endpoint occurrence, LDL-C below the estimated value of 88 mg/dL was associated with an increased risk of events (HR 0.595 [0.367–0.965], p=0.035) (Figure 5).
DiscussionThe present study evaluates the impact of on-admission LDL-C in a population of patients admitted for acute HF. In fact, a higher LDL-C level was significantly associated with better prognosis, as an increase in 1 mg/dL in LDL-C level was associated with a 4-fold increase in the likelihood of survival 1 year after discharge.
Determination of LDL-C association with prognosis in patients with high energetic demand conditions, as HF, is becoming more important. This importance arises from the fact that the most recent ESC guidelines on Dyslipidemia and Preventive Cardiology suggest even lower LDL-C targets than the previous ones.12 However, the establishment of these new targets was based on studies where HF patients may have been under-represented.10
Considering this relationship between LDL-C and mortality risk, we established an LDL-C cut-off value associated with worse outcomes. An increased risk of mortality was observed in patients with LDL-C below 88 mg/dL in the overall population. Even though this cut-off is lower than the suggested targets for moderate and low CV risk patients (100 mg/dL and 115 mg/dL, respectively), it is significantly higher than the proposed targets for high and very-high risk patients (55 mg/dL and 70 mg/dL, respectively). Moreover, as almost two thirds of the studied population presented at least high CV risk, theoretically benefiting from a stricter LDL-C control, a specific lowest LDL-C value associated with outcomes was deemed necessary for this subset of patients. The estimated value was 84 mg/dL, still significantly above the suggested LDL-C targets based on individual CV risk. It is important to stress that in this study the relationship between LDL-C and mortality was independent of HF etiology and also of individual CV risk.
We acknowledge that permitting more liberal LDL-C control in HF patients may be associated with a higher rate of thrombotic events, as higher LDL-C levels were associated with more thrombotic events in the present study. However, this trade-off may be beneficial as the net clinical benefit endpoint and mortality, principally, was positively influenced by an LDL-C level equal or above 88 mg/dL.
These results are to be considered in the light of previously published data. Rauchhaus et al., observed a significant benefit of higher total cholesterol in chronic HF survival.20 Additionally, in a study with over 1000 patients with advanced HF, a higher total cholesterol level was associated with a two-fold increase in survival likelihood.14 A long-term study with a 20-year follow-up reported unfavourable prognosis in HF patients with lower LDL-C levels, irrespective of their of the HF etiology.21 Also, in a post hoc analysis of the EVEREST study, total cholesterol and triglycerides were inversely correlated with hospitalization and mortality rates during follow-up.22 On the other hand, a cohort with 422 patients with DCM suggested that although lower cholesterol levels were associated with HF severity, a significant impact on mortality was not shown.23
Besides the direct impact on mortality, in the studied population, having an LDL-C level below the recommended target according to individual CV risk, was also associated with characteristics that are traditionally considered markers of worse prognosis or disease severity in HF patients such as increased age, lower hemoglobin or higher NT-proBNP. Also, a likelihood of higher rate of admissions was observed in patients with lower LDL-C levels. Interestingly, statin treatment did not impact LDL-C levels or the rate of patients that reached their LDL-C target according to individual CV risk. Although it is well established that by reducing ischemic events, statins lessen cardiac damage and subsequently HF development,24 similarly to previous studies,6,7 in this cohort, statins had no impact on mortality nor on other clinical outcomes. Considering all these findings, one may speculate that in HF, LDL-C levels are mostly influenced by HF related factors and not by lipid lowering therapy and, consequently, LDL-C might not be seen as a therapeutic target and instead be considered another marker of adverse prognosis in HF.24 Considering this, other CV risk markers and new metabolic targets should be sought in HF patients.
Although the nature of this relationship remains undefined, similar associations have been described in other chronic diseases such as chronic kidney disease, chronic pulmonary obstructive disease and cancer,25 as well as with advanced age.26 Different theories have been proposed. HF promotes increased metabolic demand and so one may consider LDL-C a surrogate for malnutrition or even cachexia. Although an association between low cholesterol, low albumin and worsened nutritional status had previously been reported,27 the relationship between low cholesterol levels and cachexia is inconsistent.20 Likewise, in the present cohort no relation between LDL-C level and BMI was observed and there were no differences in BMI between patients that had LDL-C below their individual recommended target and patients that did not. However, non-survivors presented a significantly lower BMI, with a median BMI of 25.0 (23.0–27.6) kg/m2, supporting the existence of obesity paradox in HF. Altogether, these data suggest that cachexia may have not played a major pathophysiological role in the impact of LDL-C in HF prognosis. Moreover, in chronic HF a consistent upregulation of inflammatory markers (C-reactive protein, TNF-α and many cytokines) is detected and associated with HF severity and poor outcomes.28,29 Considering the relationship between LDL-C and classic HF risk markers and prognosis presented in this study, one may speculate that this pro-inflammatory status may promote catabolism which, in its initial phases way before cachexia ensues, can be identified by low LDL-C levels. Another possible explanation is that congestion may promote a reduction in LDL-C both through hepatic congestion that leads to impaired lipogenesis30 and through gut edema causing cholesterol malabsorption. The fact that in the present cohort patients with LDL-C below their recommended target had higher levels of NT-proBNP supports both hypotheses.
However, these results should be interpreted in light of certain limitations. Firstly, these results derive from a single center cohort and must be externally validated, especially the LDL-C cut-off value associated with outcomes. The authors propose to act accordingly. On the other hand, this study evaluated the association with prognosis of a single determination of LDL-C and, consequently, conclusions regarding the effect of actively reducing LDL-C cannot be draw. The follow-up was limited to one-year which possibly promoted an underestimation of the association with prognosis of higher levels of LDL-C on the thrombotic events rate. Finally, most causes of death were not report and therefore not available for analysis, which prevented the establishment of any correlation between LDL-C levels and the causes of death.
ConclusionsIn conclusion, this study examined the association with prognosis of LDL-C in patients admitted for acutely decompensated HF. The findings shed light on the relationship between LDL-C levels and mortality risk in this specific population. These results demonstrated that higher LDL-C levels were associated with improved survival outcomes, with each 1 mg/dL increase in LDL-C showing a four-fold increase in the likelihood of survival one year after discharge. Importantly, the study identified the lowest LDL-C value, below which there was a markedly increased risk of mortality. For the overall population, this cut-off was determined to be 88 mg/dL, while for high and very-high CV risk patients, it was 84 mg/dL. These cut-off values are significantly higher than the recommended LDL-C targets based on individual cardiovascular risk. These findings challenge the current guidelines and raise questions about the application of standard LDL-C targets to patients with established HF. Nevertheless, these results should be externally validated, and further research is warranted to better understand the role of LDL-C in HF prognosis as well as to refine guidelines for lipid management in this specific patient population.
Conflicts of interestThe authors have no conflicts of interest to declare.