Intrinsic aortopathy can lead to dilatation late after tetralogy of Fallot (TOF) repair. Its extent and prevalence are not known. We aimed to assess aortic dimensions and elasticity and to find predictors of aortic dilatation.
MethodsA total of 126 adults were prospectively included after TOF repair and compared to 63 gender- and age-matched controls. Transthoracic echocardiography was used to assess aortic diameters at the level of the sinuses of Valsalva and ascending aorta and aortic dilatation was defined as z-score >+2. M-mode parameters of the ascending aorta were used to calculate strain, distensibility and stiffness index.
ResultsTOF patients (mean age 30±9 years; 52% male) had a complete repair at a median age of five (2-49) years; mean follow-up time since repair was 23±7 years. The prevalence of aortic dilatation at the sinuses of Valsalva and ascending aorta was 29% and 24%, respectively. Compared to controls, TOF patients had a higher ascending aorta z-score, lower strain (6.4% [0.0-61.5] vs. 15.2% [0.0-45.0], p<0.01) and higher stiffness index (7.3 [0.8-23.6] vs. 3.1 [0.9-14.1], p<0.01). On multivariate analysis male gender was strongly associated with sinuses of Valsalva dilatation (odds ratio 6.3, 95% confidence interval 1.5-26.3, p=0.01).
ConclusionsThe prevalence of aortic dilatation late after TOF repair is significant, with a larger and stiffer ascending aorta. Male gender appears to influence aortic root dilatation. This aortopathy requires careful follow-up in order to prevent future complications.
Uma possível aortopatia intrínseca poderá condicionar dilatação tardia da aorta na tetralogia de Fallot operada. A sua prevalência e extensão não estão definidas. Pretendemos avaliar as dimensões e a elasticidade da aorta e encontrar preditores da dilatação aórtica.
MétodosIncluímos prospetivamente 126 adultos operados a tetralogia de Fallot e comparamos com 63 controlos. Avaliamos por ecocardiografia transtorácica os diâmetros dos seios de Valsalva e da aorta ascendente e definimos dilatação aórtica para z-score > +2. Parâmetros modo M da aorta ascendente foram usados para calcular strain, distensibilidade e índice de rigidez.
ResultadosDoentes com tetralogia de Fallot (idade média 30 ± 9 anos; 52% homens) foram operados com uma idade mediana de 5 (2-49) anos; tempo médio de seguimento desde a cirurgia 23 ± 7 anos. A prevalência de dilatação dos seios de Valsalva e da aorta ascendente foi 29% e 24%, respetivamente. Comparado aos controlos, os doentes com tetralogia de Fallot apresentaram maior z-score da aorta ascendente, menor strain (6,4 [0,0-61,5] versus 15,2 [0,0-45,0]%; p < 0,01) e maior índice de rigidez (7,3 [0,8-23,6] versus 3,1 [0,9-14,1]; p < 0,01). Na análise multivariada o sexo masculino associou-se significativamente à dilatação dos seios de Valsalva (odds ratio 6,3, intervalo de confiança de 95% 1,5-26,3; p = 0,01).
ConclusõesNa tetralogia de Fallot operada há uma prevalência significativa de dilatação tardia da aorta, com a aorta ascendente maior e mais rígida. O sexo masculino parece influenciar a dilatação da raiz da aorta. Esta aortopatia requer um seguimento cuidadoso para evitar complicações futuras.
two‐dimensional
ascending aorta
ascending aorta z-score
aortic diastolic diameter
aortic
Ao root z-score
aortic systolic diameter
blood pressure
body surface area
confidence interval
distensibility
diastolic blood pressure
grown-up congenital heart
left ventricular
left ventricular ejection fraction
odds ratio
pulse pressure
systemic arterial compliance
systolic blood pressure
standard deviation
sinuses of Valsalva
sinotubular junction
stroke volume
stroke volume index
tetralogy of Fallot
transthoracic echocardiography
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease in which survival into adulthood is common. The guidelines recommend regular long-term follow-up in specialized grown-up congenital heart (GUCH) centers.1
Major residual lesions after TOF repair occur at the level of the right ventricular outflow tract, including obstruction or aneurysm, and at the pulmonary valve due to regurgitation or stenosis. In addition, as first described by Capelli et al.,2 there is an increasing awareness that aortic (Ao) dilatation can develop late after TOF repair. Interestingly, Ao dilatation is a fetal feature of TOF that tends to disappear after early surgical repair3 without a palliative systemic-to-pulmonary shunt, thus preventing long-standing volume overload on the overriding aorta. Ao dilatation may be a disorder of the past, not reflecting the current surgical era.4 Nevertheless, for many reasons, including anatomical and technical surgical issues, not all patients will benefit from early repair. Additionally, aortic histological abnormalities present since infancy in TOF, especially in the Ao root and ascending aorta (AAo) vascular wall, can contribute to Ao dilatation.5,6 This intrinsic aortopathy can lead to Ao dilatation late after TOF repair, but its extent and prevalence are not known. We aimed to assess proximal thoracic aorta dimensions and elasticity by transthoracic echocardiography, in TOF patients and normal controls, and to find possible predictors of Ao dilatation.
MethodsStudy populationInclusion criteriaWe prospectively included 126 adults (age ≥18 years) with repaired TOF, without pulmonary atresia or absent pulmonary valve, followed at our GUCH center between March 2011 and December 2013, and 63 gender- and age-matched healthy controls.
Exclusion criteriaWe excluded 21 patients: three with associated heart disease (one Ao valve stenosis and two Ao mechanical prosthesis), 13 with genetic syndromes (11 Down syndrome and two DiGeorge syndrome), and five due to pregnancy.
Study protocolSystemic arterial hemodynamicsArterial blood pressure (BP) (systolic [SBP] and diastolic [DBP]) was measured twice with a 2-min interval in supine position and averaged, using an automated blood pressure monitor (SureSigns VS2, Philips Medical Systems). We chose to measure BP in the left brachial artery, due to the presence of a previous right Blalock-Taussig shunt in a significant number of cases (49 patients). If there was a palliative left shunt, BP was measured in the right arm (nine cases).
The ratio of stroke volume index (SVI) to pulse pressure (PP) was used as an indirect measure of total systemic arterial compliance (SAC=SVI/PP).7
Arterial stiffness was calculated as 0.9×SBP/stroke volume (SV).8
M-mode, two-dimensional and Doppler echocardiographic examinationTransthoracic echocardiography (TTE) was performed immediately after BP measurement using a Vivid E7 ultrasound system (GE Healthcare, Milwaukee, WI, USA) with an M4S sector array transducer (1.5-4.3MHz) and digitally stored. All echocardiographic exams were performed and analyzed by an experienced cardiologist, certified in congenital heart disease echocardiography by the European Association of Cardiovascular Imaging and blinded to patient data.
Aortic assessmentAo diameters were measured by two-dimensional (2D) TTE at the level of the sinuses of Valsalva (SoV) and sinotubular junction (STJ) and at the proximal AAo, 2-3cm above the STJ, in parasternal long-axis view perpendicular to the Ao long axis, using the maximum leading edge-to-leading edge diameter method at end-diastole, and were indexed to body surface area (BSA) according to the DuBois formula.9
The Ao sinus z-score (AoZ) was obtained using the standard nomograms for Ao root size in healthy adults, adapted from Roman et al.10 The AAo z-score (AAoZ) was based on reference values and equations from Campens et al.11 Ao dilatation was defined as a z-score >+2, for both the SoV and AAo.
Elastic properties of the ascending aortaAAo distensibility (D) was calculated as 2(As-Ad)/[Ad(SBP-DBP)] in KPa-1×103, and M-mode derived stiffness index (β1) was calculated as ln(SBP/DBP)/(As-Ad)/Ad.
As and Ad were obtained using the M-mode inner systolic and diastolic AAo diameters in short-axis view, at the level of the pulmonary artery bifurcation, with Ad obtained at the peak of the R wave of the simultaneously recorded electrocardiogram, while As was measured at maximum anterior AAo wall motion.12
Left ventricular assessmentLeft ventricular (LV) end-diastolic and end-systolic inner diameters, posterior wall and septal thickness were measured by 2D-TTE in parasternal long-axis view.
LV mass was calculated by the corrected formula of the American Society of Echocardiography. Left venricular ejection fraction (LVEF) was calculated using the modified Simpson's rule.13 SV was obtained by subtracting end-systolic volume from end-diastolic volume, using 2D volumetric measurements for the LVEF calculation. LV measurements were indexed to BSA according to the DuBois formula.9 Pulsed-wave tissue Doppler was used to estimate LV filling pressure by calculating the E/lateral e’ ratio.14 Septal e’ was not used because in TOF, following patch closure of the ventricular septal defect, septal e’ is underestimated and is therefore not helpful in determining diastolic function.
EthicsThe study protocol complies with the Declaration of Helsinki. It was approved by the local institutional ethics committee and written informed consent was obtained from all patients.
Statistical analysisThe statistical analysis was performed using IBM SPSS Statistics, version 24.0 (IBM SPSS Inc., Chicago, IL, USA). A two-tailed p-value <0.05 was considered statistically significant. Continuous data are expressed as mean ± standard deviation (SD) or as median (range), according to the normality of the variable's distribution. Categorical variables are summarized as frequency and percentage. The normality of the data was determined with histograms and using the Kolmogorov-Smirnov or Shapiro-Wilk test, as appropriate. A paired Student's t test or a Mann-Whitney U test, as appropriate, was performed to compare continuous variables between patients and controls. Intra- and interobserver variability were assessed in 18 randomly selected cases. Bland-Altman analysis was used to determine bias (mean of the difference) with 95% limits of agreement. Pearson's correlation coefficient was used to analyze possible associations between the AAo z-score and a number of continuous variables. Variables associated with Ao dilatation (SoV and AAo z-score >+2) were assessed in univariate and multivariate logistic regression models.
ResultsDemographic and echocardiographic characteristics of the study population are shown in Table 1. A total of 126 TOF patients were included (mean age 30±9 years; 52% male), with a mean follow-up since TOF repair of 23±7 years. Most were asymptomatic (91%). A right Ao arch was present in 29 patients. A previous systemic-to-pulmonary shunt had been performed in 58 cases, with a median interval of three (1-22) years. The shunt-to-repair time was ≥5 and ≥10 years in 12 and two patients, respectively. Surgical repair was performed in adulthood (age ≥18 years) in 12 patients. A transannular patch was used for repair in 57 patients. No differences were found between TOF patients and controls regarding pulse pressure, LV mass index or LV stroke volume index. AAo diameter could be measured in 121 patients; it was larger in TOF patients (33.8±4.8 vs. 26.1±3.1mm, p<0.01), and the STJ was effaced in 53 cases. AAo was larger than SoV in 40% (48/121) of TOF patients. Compared to controls, TOF patients had higher AoZ and AAoZ, lower AAo strain (6.4% [0.0-61.5] vs. 15.2% [0.0-45.0], p<0.01) and higher AAo stiffness index (7.3 [0.8-23.6] vs. 3.1 [0.9-14.1], p<0.01). Comparison between TOF patients according to AAo z-score is shown in Table 2. The prevalence of Ao dilatation at the SoV and AAo (AoZ and AAoZ >+2) was 29% and 24%, respectively, with a maximum absolute SoV ≥45mm in five cases and AAo ≥45mm in four cases. As expected, patients with AAo dilatation (AAo z-score >+2) had a significantly higher BSA, but they had larger LV mass and volumes even after indexing for BSA. Importantly, patients with AAo dilatation had undergone later TOF repair, with a similar median palliative shunt-to-repair time. Patients without AAo dilatation had undergone early repair but the proportion receiving a transannular patch for surgical repair was significantly higher.
Demographic and echocardiographic characteristics of the study population.
| TOF (n=126) | Controls (n=63) | p | |
|---|---|---|---|
| Age, years | 30.2±9.2 | 31.4±10.1 | 0.42 |
| Male gender, n (%) | 66 (52.4) | 33 (52.4) | 1.00 |
| Body surface area, m2 | 1.7±0.2 | 1.7±0.2 | 0.22 |
| Systemic arterial hemodynamics | |||
| Systolic blood pressure, mmHg | 117.5±11.8 | 115.8±10.1 | 0.36 |
| Diastolic blood pressure, mmHg | 70.7±8.8 | 70.0±8.7 | 0.65 |
| Pulse pressure, mmHg | 46.8±8.0 | 45.8±6.3 | 0.38 |
| Arterial stiffness, mmHg/mla | 2.0±0.6 | 1.9±0.5 | 0.33 |
| SAC, ml/m2/mmHga | 0.7±0.2 | 0.7±0.2 | 0.73 |
| Aortic measurements | |||
| SoV diameter, mm | 34.0±4.7 | 28.0±3.2 | <0.01 |
| SoV index, mm/m2 | 19.9±2.3 | 16.2±2.1 | <0.01 |
| SoV z-score | 1.4±1.2 | -0.6±1.2 | <0.01 |
| STJ diameter, mm | 30.7±4.2 | 23.8±3.1 | <0.01 |
| AAo diameter, mm | 33.8±4.8 | 26.1±3.1 | <0.01 |
| AAo index, mm/m2 | 19.9±2.6 | 15.0±2.1 | <0.01 |
| AAoZ | 1.2±1.1 | -0.7±0.7 | <0.01 |
| LV study | |||
| LV mass index, g/m2 | 79.8±20.7 | 78.9±15.2 | 0.74 |
| LV mass/volume ratio, g/ml | 1.6±0.5 | 1.5±0.4 | 0.14 |
| LVEDV index, ml/m2 | 51.2±12.3 | 54.1±10.7 | 0.12 |
| LVESV index, ml/m2a | 18.9±6.3 | 18.9±6.2 | 0.99 |
| LV stroke volume index, ml/m2a | 33.4±8.6 | 33.8±7.8 | 0.79 |
| LVEF, %a | 64.1±7.0 | 64.5±6.4 | 0.71 |
| E/E’ ratio | 5.5±1.9 | 4.4±0.9 | <0.01 |
| Aortic elastic properties | |||
| Aortic strain, %a | 6.4 (0.0-61.5) | 15.2 (0.0-45.0) | <0.01 |
| Aortic distensibility, KPa-1×103a | 1.3 (0.0-14.0) | 3.4 (0.0-12.5) | <0.01 |
| Aortic stiffness indexa | 7.3 (0.8-23.6) | 3.1 (0.9-14.1) | <0.01 |
Data are expressed as mean ± standard deviation (SD) or median (range). Categorical variables are summarized as frequency and percentage. Aortic diameters are measured in mm.
AAo: ascending aorta; AAoZ: ascending aorta z-score; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; SAC: systemic arterial compliance; SoV: sinus of Valsalva; STJ: sinotubular junction; TOF: tetralogy of Fallot.
Comparison between tetralogy of Fallot patients according to z-score.
| AAo z-score ≤+2 (n=92) | AAo z-score >+2 (n=29) | p | |
|---|---|---|---|
| Age, years | 28.9±8.0 | 34.9±11.7 | 0.01 |
| Male gender, n (%) | 45 (48.9) | 19 (65.5) | 0.12 |
| Body surface area, m2 | 1.68±0.2 | 1.82±0.2 | <0.01 |
| Right aortic arch, n (%) | 20 (21.7) | 9 (31.0) | 0.31 |
| Transannular patch, n (%) | 47 (51.1) | 7 (24.1) | 0.02 |
| Previous palliative shunt, n (%) | 46 (50.0) | 9 (31.0) | 0.07 |
| Age at repair, years | 5 (2-39) | 7 (2-49) | 0.03 |
| Shunt-to-repair time | 3 (1-22) | 3 (1-11) | NS |
| Follow-up since repair | 23.3±5.4 | 23.6±10.0 | 0.87 |
| Aortic regurgitation grade III-IV, n (%) | 0 (0.0) | 2 (6.9) | 0.06 |
| Systemic hypertension, n (%) | 5 (5.4) | 3 (10.3) | 0.40 |
| Systemic arterial hemodynamics | |||
| SBP, mmHg | 117.0±12.0 | 119.6±11.3 | 0.32 |
| DBP, mmHg | 69.9±8.6 | 72.6±10.0 | 0.16 |
| Pulse pressure, mmHg | 47.1±8.0 | 46.7±8.1 | 0.82 |
| Arterial stiffness, mmHg/mla | 2.0 (1.1-3.5) | 1.7 (0.9-2.8) | NS |
| SAC, ml/m2/mmHga | 0.7 (0.3-1.4) | 0.7 (0.4-1.5) | NS |
| Aortic measurements | |||
| SoV diameter, mm | 32.6±4.0 | 38.6±4.1 | <0.01 |
| SoV index, mm/m2 | 19.6±2.4 | 21.3±1.6 | <0.01 |
| SoV z-score | 1.2±1.2 | 2.2±0.7 | <0.01 |
| STJ diameter, mm | 30.0±3.7 | 34.6±5.2 | <0.01 |
| AAo diameter, mm | 31.8±3.0 | 40.2±3.3 | <0.01 |
| AAo index, mm/m2 | 19.1±2.4 | 22.2±1.6 | <0.01 |
| AAoZ | 0.7±0.7 | 2.6±0.6 | <0.01 |
| LV study | |||
| LV mass index, g/m2 | 76.9±18.6 | 90.4±23.7 | <0.01 |
| LV mass/volume ratio, g/ml | 1.6±0.5 | 1.6±0.4 | 0.92 |
| LVEDV index, ml/m2 | 50.0±12.7 | 55.7±10.6 | 0.04 |
| LVESV index, ml/m2a | 18.3±6.1 | 21.3±6.5 | 0.04 |
| LV stroke volume index, ml/m2a | 32.9±8.3 | 35.0±9.6 | 0.29 |
| LVEF, %a | 64.3±6.9 | 62.2±6.5 | 0.18 |
| Aortic elastic properties | |||
| Aortic strain, %a | 6.9 (0.0-40.0) | 5.0 (0.0-61.5) | NS |
| Aortic distensibility, KPa-1×103a | 1.4 (0.0-8.9) | 1.0 (0.0-14.0) | NS |
| Aortic stiffness indexa | 7.2 (1.2-23.2) | 9.9 (0.8-23.6) | NS |
Data are expressed as mean ± standard deviation (SD) or median (range). Categorical variables are summarized as frequency and percentage. Aortic diameters are measured in mm.
AAo: ascending aorta; AAoZ: ascending aorta z-score; DBP: diastolic blood pressure; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; SAC: systemic arterial compliance; SBP: systolic blood pressure; SoV: sinus of Valsalva; STJ: sinotubular junction; TOF: tetralogy of Fallot.
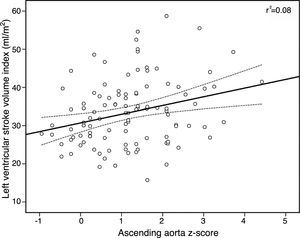
There was a significant, albeit modest, positive correlation between AAoZ and SAC (Pearson's correlation [r]=0.24, p=0.01) and a negative correlation with arterial stiffness (r=-0.27, p=0.008), but not with AAo strain or stiffness index. There was also a modest but significant correlation between AAoZ and LV mass index (r=0.29, p=0.001) and SVI (r=0.28, p=0.004) (Figure 1). On multivariate analysis (Tables 3 and 4), male gender was strongly associated with SoV dilatation (odds ratio [OR] 6.3, 95% confidence interval [CI] 1.5-26.3, p=0.01), but not with AAo dilatation. Instead, AAo/SoV ratio >1 showed the strongest link with AAo dilatation (OR 5.3, 95% CI 1.3-20.9, P=0.02).
Logistic regression analysis according to sinus of Valsalva dilatation (z-score >+2).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR | p | OR | p | |
| Age | 1.03 | 0.18 | 0.98 | 0.57 |
| Gender (male) | 2.07 | 0.07 | 6.27 | 0.01 |
| Right aortic arch | 0.56 | 0.25 | ||
| Previous palliative shunt | 0.53 | 0.12 | 0.71 | 0.62 |
| Transannular patch | 0.49 | 0.08 | 0.36 | 0.13 |
| Time to surgical repair | 1.02 | 0.43 | ||
| Time since repair | 1.00 | 0.91 | ||
| AAoZ | 3.38 | <0.01 | ||
| STJ effacement | 1.14 | 0.75 | ||
| AAo/SoV ratio >1 | 0.51 | 0.12 | 0.16 | 0.01 |
| LV mass index | 1.03 | 0.01 | 1.05 | 0.01 |
| LV mass/volume ratio | 1.08 | 0.86 | ||
| LVEDV index | 1.05 | <0.01 | ||
| LVESV index | 1.06 | 0.08 | ||
| LV stroke volume index | 1.07 | 0.01 | 1.17 | 0.07 |
| LVEF | 1.00 | 0.94 | ||
| SBP | 1.01 | 0.54 | ||
| DBP | 1.03 | 0.22 | 1.04 | 0.30 |
| Pulse pressure | 1.00 | 0.58 | ||
| Arterial stiffness | 0.36 | 0.02 | 4.08 | 0.34 |
| SAC | 9.62 | 0.02 | 0.55 | 0.83 |
| Ao strain | 1.00 | 0.66 | ||
| Ao distensibility | 0.00 | 0.63 | ||
| Ao stiffness index | 1.04 | 0.32 |
AAo: ascending aorta; AAoZ: ascending aorta z-score; Ao: aortic; DBP: diastolic blood pressure; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; OR: odds ratio; SAC: systemic arterial compliance; SBP: systolic blood pressure; SoV: sinus of Valsalva; STJ: sinotubular junction.
Logistic regression analysis according to ascending aorta dilatation (z-score >+2).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR | p | OR | p | |
| Age | 1.07 | <0.01 | 1.00 | 0.98 |
| Gender (male) | 1.98 | 0.12 | 0.75 | 0.66 |
| Right aortic arch | 1.62 | 0.31 | ||
| Previous palliative shunt | 0.45 | 0.08 | 1.02 | 0.98 |
| Transannular patch | 0.33 | 0.02 | 0.19 | 0.03 |
| Time to surgical repair | 1.06 | 0.03 | 1.03 | 0.53 |
| Time since repair | 1.01 | 0.82 | ||
| STJ effacement | 3.24 | <0.01 | 4.67 | 0.03 |
| AAo/SoV ratio >1 | 4.13 | <0.01 | 5.28 | 0.02 |
| LV mass index | 1.03 | <0.01 | 1.03 | 0.14 |
| LV mass/volume ratio | 1.05 | 0.92 | ||
| LVEDV index | 1.04 | 0.04 | 0.99 | 0.83 |
| LVESV index | 1.08 | 0.05 | 1.02 | 0.76 |
| LV stroke volume index | 1.03 | 0.28 | ||
| SBP | 1.02 | 0.31 | ||
| DBP | 1.04 | 0.16 | 1.10 | 0.03 |
| Pulse pressure | 0.99 | 0.82 | ||
| Arterial stiffness | 0.54 | 0.16 | 0.89 | 0.91 |
| SAC | 4.56 | 0.12 | 5.92 | 0.46 |
| Ao strain | 1.00 | 0.90 | ||
| Ao distensibility | 7.65 | 0.82 | ||
| Ao stiffness index | 1.02 | 0.56 | ||
AAo: ascending aorta; AAoZ: ascending aorta z-score; Ao: aortic; DBP: diastolic blood pressure; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; OR: odds ratio; SAC: systemic arterial compliance; SBP: systolic blood pressure; SoV: sinus of Valsalva; STJ: sinotubular junction.
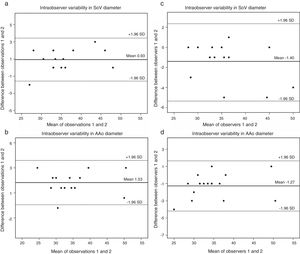
A sample of 15 randomly chosen cases were reanalyzed. Intraobserver variability was 0.93mm (95% CI 0.22 to 1.64) (Figure 2a) for the SoV diameter and 1.53mm (95% CI -0.68 to 3.74) (Figure 2b) for AAo diameter. Interobserver variability was -1.40mm (95% CI -2.46 to -0.34) (Figure 2c) for the SoV and -1.27mm (95% CI -4.19 to 1.65) for Aao diameters, respectively (Figure 2d).
(a) Bland-Altman plot of intraobserver variability in SoV diameter measurements (in mm). Estimated bias: 0.93% (95% confidence interval 0.22 to 1.64); (b) Bland-Altman plot of intraobserver variability in AAo diameter (in mm). Estimated bias 1.53% (95% confidence interval -0.68 to 3.74); (c) Bland-Altman plot of interobserver variability in SoV diameter (in mm). Estimated bias -1.40mm (95% confidence interval -2.46 to -0.34); (d) Bland-Altman plot of interobserver variability in AAo diameter (in mm). Estimated bias -1.27% (95% confidence interval -4.19 to 1.65mm). AAo: ascending aorta; SD: standard deviation; SoV: sinuses of Valsalva.
Ao dilatation can occur in adults after TOF repair but its prevalence varies according to the study population, the Ao segment analyzed and the cut-off value used for definition. Niwa et al. reported 15% Ao root dilatation using an observed-to-expected cut-off >1.5 at the STJ.15 A multicenter cross-sectional study found a prevalence of 7% using an observed-to-expected ratio >1.5 at the SoV,16 while Nagy et al. found a prevalence of 51% using an SoV z-score >+2.17 In contrast to previous studies, we decided to exclude pulmonary atresia and TOF with absent pulmonary valve in order to avoid a population bias, since in both anatomical variants Ao regurgitation and Ao root dilatation are more common after repair. Moreover, pulmonary atresia is frequently associated with a complex pulmonary vascular pattern and variable major aortopulmonary collaterals, with consequent LV volume overload and a higher prevalence of Ao dilatation.18 A single-center study19 also excluded pulmonary atresia and found Ao root dilatation (≥40mm) in 24% of patients, similar to our results. In our study there was no association of right Ao arch and Ao dilatation in TOF, probably due to the exclusion of pulmonary atresia.20 As stated above, this suggests that it is Ao volume overload that predisposes to Ao dilation and not the sidedness of the Ao arch.4
We decided to exclude genetic syndromes, including Down and DiGeorge syndromes, due to their frequent association with developmental delay, especially growth retardation, in order to avoid influencing study results when comparing TOF patients with normal controls (e.g. indexed Ao dimension or Ao z-score). Unfortunately, we cannot exclude the possible existence of partial 22q11 deletion in non-syndromic patients, as genetic testing was not performed systematically in all patients.
Although absolute Ao diameters are used for surgical decisions,21,22 z-scores that adjust Ao dimensions for age and BSA appear to be more accurate for definition and longitudinal follow-up in congenital heart disease. Of note, the fact that Ao dilatation included not only the Ao root but also the AAo highlights the importance of complete Ao assessment late after TOF repair. We found a significant prevalence of Ao dilatation, partially explained by a referral bias to a GUCH center, but this was a real-world adult TOF population, reflecting the patients’ historical medical, surgical and social background. Although this is rare, some patients are only diagnosed in adulthood. In our cohort 10% of patients had undergone surgical repair at age 18 or older. As previously reported, later TOF repair leads to longer Ao volume overload, which has an effect on late Ao dilatation. In our study, patients with AAo dilatation had also undergone later TOF repair, but with a similar median palliative shunt-to-repair time, compared to patients without AAo dilatation. More advanced age at surgical shunt or repair was found mostly in our historical older patients, with late referral for both medical and surgical care, which is not seen in contemporary TOF patient populations. In addition, only a small number of patients with AAo dilatation had a palliative shunt prior to repair, and this could have influenced the statistical analysis.
Similarly to Chong et al.,23 who used M-mode strain in a pediatric population, we found abnormal AAo elasticity in adults. Reduced AAo strain in TOF patients could be an expression of abnormal AAo elasticity, a consequence of histological changes, and not simply a reflection of Ao dilatation, which would explain the absence of a significant correlation with AAoZ. We found no correlation between M-mode AAo strain and SAC or arterial stiffness. This lack of association may be explained by the patients’ young age (<30 years in 54%), and by the fact that M-mode AAo strain is a local elastic measurement, and may not reflect overall systemic arterial elasticity. Applanation tonometry, as the gold standard method for assessing arterial stiffness, could help clarify whether aortopathy in TOF has a real impact on systemic arterial stiffness. Nevertheless, in TOF patients with AAo dilatation we found increased LV mass and volumes and a small correlation between AAoZ and LV mass index. It could be speculated that Ao dilatation with reduced Ao strain could increase afterload and influence LV remodeling. Further studies are needed to test this hypothesis.
Male gender appears to be the most consistent predisposing factor for Ao dilatation. As in previous studies,15,16,24 male gender had a strong association with SoV dilatation. Even though predictors of Ao dilatation found in the literature, such as prior palliative shunt, late TOF repair and longer shunt-to-surgical repair time,3,15 suggest that long-standing Ao volume overload prior to repair can lead to dilatation, in our study shunt-to-repair time and age at surgery were not predictors of Ao dilatation. This discrepancy could be partly attributed to the exclusion of pulmonary atresia, the sample size and the advanced age at repair of a significant number of patients, with and without Ao dilatation. We should emphasize that, apart from the Ao volume overload trigger, other underlying etiologies such as intrinsic histological and elastic changes may contribute to abnormal Ao deformation.
Ao dissection late after TOF repair has been reported, the longest interval being 46 years,25 and in one case dissection was diagnosed at the age of 18 years, even though TOF repair had been performed in infancy at the age of two months.26 Additionally, some patients need Ao root replacement during follow-up, most due to Ao regurgitation.27 In our center, since the beginning of the study in 2011 to date two patients have been scheduled for a Bentall operation in addition to pulmonary valve replacement (unpublished data). These findings highlight the need for careful long-term follow-up in order to prevent Ao complications in the future. It remains to be seen whether early TOF repair in infancy will prevent late Ao dilatation.
Study limitationsThe first limitation relates to the sample size and selection of patients referred to a single tertiary care center, which may have led to selection bias. However, the healthy control group was age- and gender-matched.
Secondly, TTE can be challenging in GUCH patients, due to a poor acoustic window. Nevertheless, SoV and AAo could be assessed in the vast majority of patients, but SV or LVEF using Simpson's rule was assessed in 110 cases. A multimodality imaging approach could perhaps increase the accuracy of LV volumetric variables. Echocardiographic data were assessed offline and the reviewer only had access to short-axis views of the AAo for M-mode and 2D reanalysis, but we cannot exclude the possibility that the reviewer was able to identify cases of TOF.
Thirdly, we used brachial artery BP instead of central Ao pressure, and since this could have overestimated DBP, the assessment of Ao distensibility may also have been overestimated. However, the distensibility of the AAo measured by non-invasive methods is close to that obtained by direct measurements.12 Also, PP amplification, especially in young individuals, may have led to overestimation of Ao stiffness when using brachial PP,28 but this applies to the entire cohort and did not change the results when comparing TOF patients to controls.
Longitudinal imaging studies of the thoracic aorta in a larger population late after TOF repair are needed to find predictors that could be used to screen for and prevent adverse Ao outcomes, including dissection.
ConclusionsTetralogy of Fallot patients have a significant prevalence of Ao dilatation late after repair, with a larger and stiffer ascending aorta. This aortopathy requires careful follow-up in order to prevent future complications.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank all volunteers, including medical students, staff technicians and nurses, who participated in this study.










 SoV diameter measurements (in mm). Estimated bias: 0.93% (95% confidence interval 0.22 to 1.64); (b) Bland-Altman plot of intraobserver variability in
SoV diameter measurements (in mm). Estimated bias: 0.93% (95% confidence interval 0.22 to 1.64); (b) Bland-Altman plot of intraobserver variability in 

