With the development of interventional procedures, iatrogenic aorto-right ventricular fistulae are increasingly reported. They may follow surgical aortic valve replacement or percutaneous aortic valve implantation, leading to high morbidity. Traditionally, treatment of fistulae was based on surgical repair, but with advances in endovascular technologies, more emphasis is now placed on percutaneous closure.
We report the case of a 78-year-old patient with severe symptomatic aortic stenosis who underwent surgical aortic valve replacement with a Perceval sutureless valve. One month later, he presented with symptoms and signs of heart failure. Transthoracic and transesophageal echocardiography confirmed the presence of a aorto-right ventricular fistula. The fistula was successfully closed percutaneously with an Amplatzer Vascular Plug II, in an intracardiac echocardiography-guided procedure.
Aorto-right ventricular fistula is a rare finding after surgical aortic valve replacement and to our knowledge it has never been associated with sutureless aortic valve replacement. A percutaneous procedure with an appropriately selected device may be encouraged because of the high morbidity and mortality of redo open-heart surgery. To minimize the risk of a second general anesthesia, the use of intracardiac echocardiography to guide the percutaneous procedure is feasible and safe.
Com o desenvolvimento de procedimentos invasivos tem havido um aumento da documentação de fistulas iatrogénicas da aorta para o ventrículo direito. Estas podem ocorrer após implantação de válvula aórtica cirúrgica ou percutânea e estão associadas a elevada morbilidade. Tradicionalmente o tratamento desta complicação é cirúrgico, mas, com o desenvolvimento de técnicas endovasculares, um maior enfâse tem sido dado ao encerramento percutâneo.
Reportamos um caso de um doente de 78 anos com estenose aórtica grave sintomática tratado cirurgicamente com implantação de uma válvula Perceval sutureless. Um mês após a intervenção, recorreu ao serviço de urgência por sintomas e sinais de insuficiência cardíaca. A avaliação ecocardiográfica transtorácica e transesofágica confirmou a presença de uma fístula da aorta para o ventrículo direito. A fístula foi encerrada percutaneamente com sucesso, utilizando um Amplatzer Vascular Plug II, num procedimento guiado por ecocardiográfica intracavitária.
O desenvolvimento de uma fístula da aorta para o ventrículo direito é raro após uma cirurgia de substituição valvular aórtica, não existindo casos descritos do seu aparecimento após implantação de uma válvula sem sutura. Nestes casos, o encerramento percutâneo com um dispositivo adequado deve ser encorajado, evitando a morbilidade e mortalidade associadas a um novo procedimento cirúrgico. Para minimizar o risco associado a anestesia geral, guiar o procedimento por ecocardiograma intracavitário é possível e seguro.
Fistulae between the aorta and the right ventricle are rare and are usually associated with trauma, rupture of the sinus of Valsalva or infective endocarditis.1,2 However, with the development of interventional procedures, iatrogenic aorto-right ventricular (aorto-RV) fistulae are increasingly reported. They may follow either surgical aortic valve replacement (SAVR)3 or percutaneous aortic valve implantation (TAVI).4 These pathologic communications have a high morbidity and can lead to hemodynamic instability and heart failure.
Traditionally, treatment of aorto-RV fistulae was based on surgical repair.1 With advances in endovascular technologies, more emphasis is now placed on percutaneous closure. These procedures are usually guided by transesophageal echocardiography (two- or three-dimensional) under general anesthesia. More recently, intracardiac echocardiography (ICE) has increasingly been used, and is currently a helpful tool for interventional cardiologists in different procedures. Experience of ICE guidance for percutaneous device closure of left-to-right shunts (other than intra-atrial septal defect), although attractive, is limited, with published evidence only regarding closure of ventricular septal defects and patent ductus arteriosus.5,6
We describe a case of aorto-RV fistula post-SAVR following aortic valve replacement with a Perceval sutureless valve (LivaNova PLC, London, UK). The fistula was successfully closed with percutaneous implantation of an Amplatzer Vascular Plug II (Abbott Vascular, USA). To our knowledge, this is both the first reported case of a aorto-RV fistula developing after a sutureless aortic valve prosthesis implantation and also the first report of percutaneous closure of an aorto-RV fistula guided by ICE.
Case reportA 78-year-old man sought care at the emergency department for acute onset of orthopnea, dyspnea and peripheral edema. Twelve days before, he had undergone SAVR with a 23 mm Perceval sutureless valve, combined with coronary artery bypass grafting (left internal mammary artery to left anterior descending artery) and Morrow myectomy. His postoperative course was complicated with complete heart block resolved with permanent pacemaker implantation.
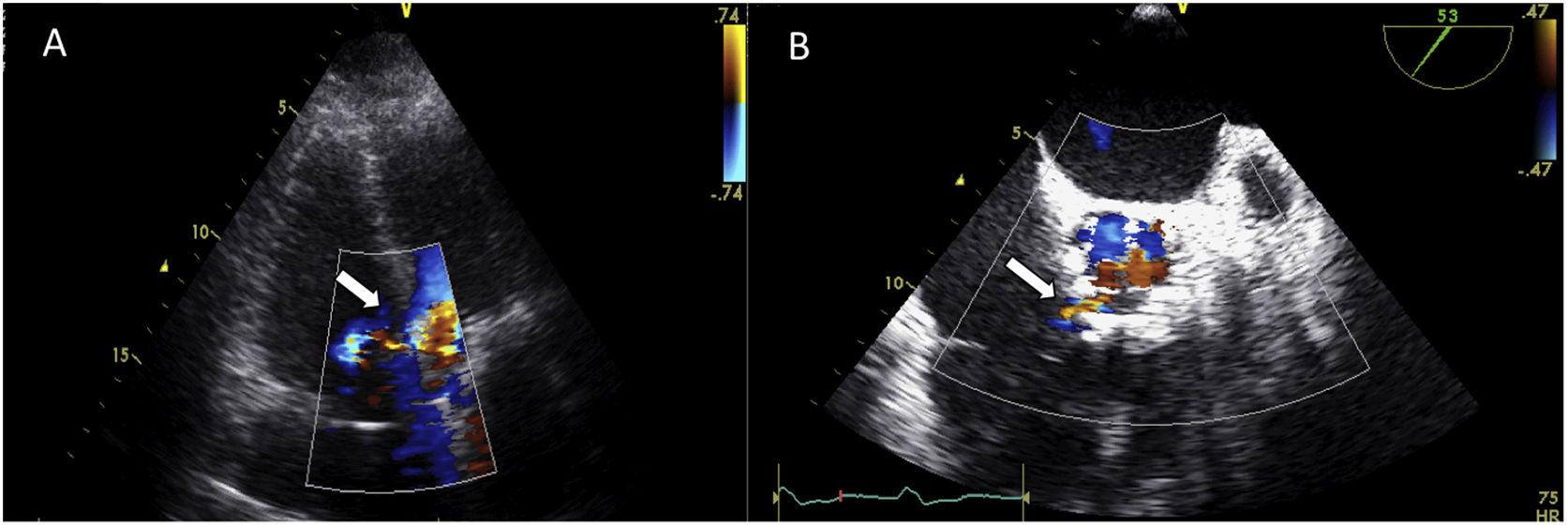
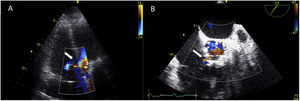
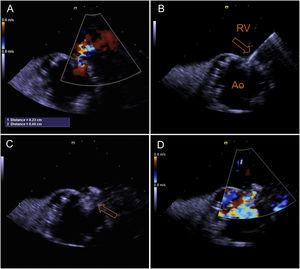
On initial assessment his complete blood cell count and serum electrolyte levels were within normal limits. NT-proBNP level was 5030 pg/ml. There was no evidence of infective endocarditis. Chest radiography showed an increased cardiothoracic ratio with pulmonary vascular congestion. Transthoracic echocardiography revealed normal left ventricular function and normal functioning of the prosthetic aortic valve, dilatation of the right chambers with right ventricular systolic dysfunction (gradient between right ventricle and atrium of 40 mmHg), and a continuous color-flow jet from the aorta toward the right ventricular cavity (Figure 1A). Transesophageal echocardiography (TEE) confirmed the existence of a shunt between the aorta (at the level of the native right cusp) and the right ventricle (immediately above the insertion of the tricuspid annulus) (Figure 1B). It also ruled out infective endocarditis behind this abnormal communication and showed a well-seated prosthetic valve with no vegetation, prosthetic abscess, or aneurysm in the aortic root.
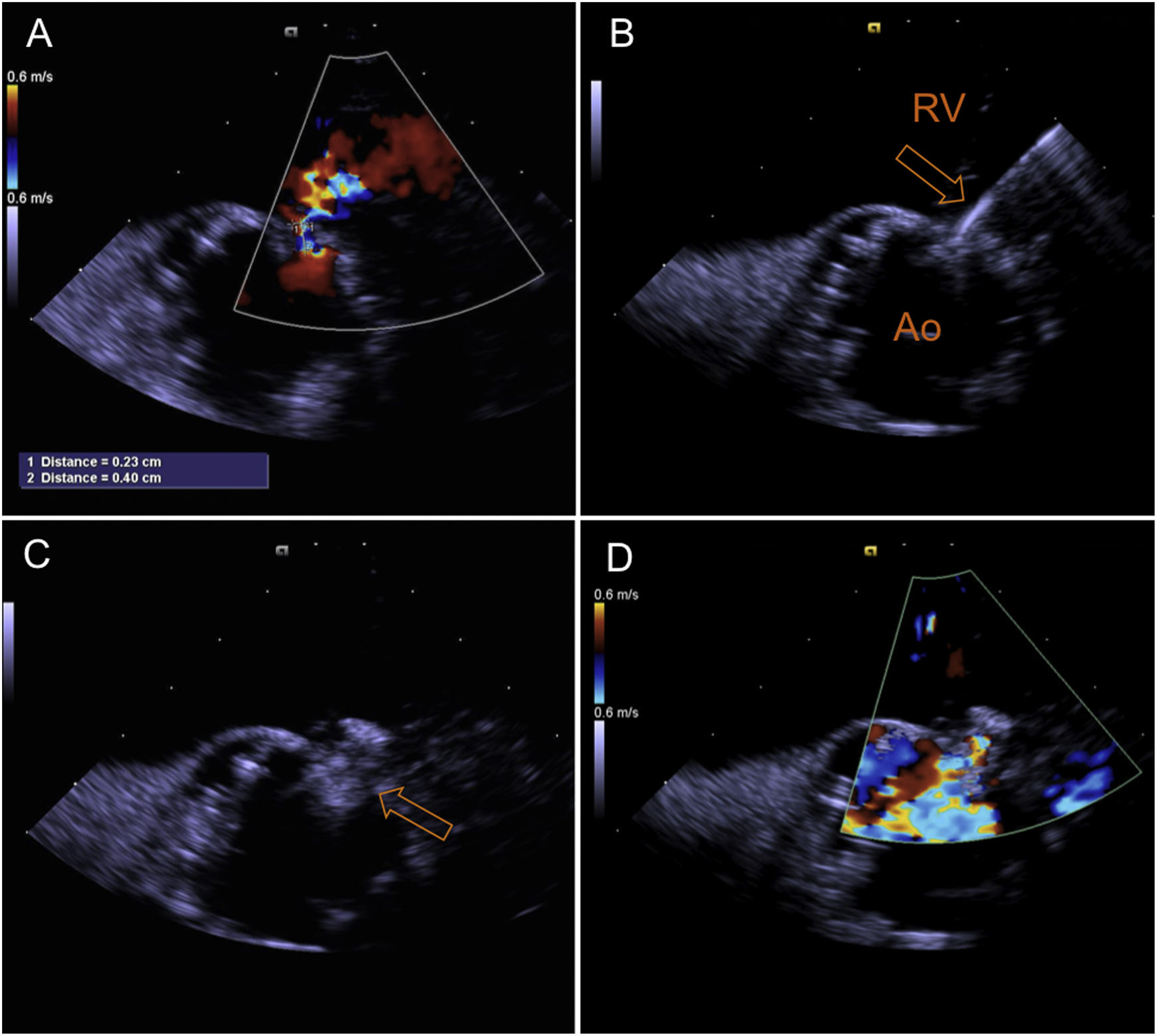
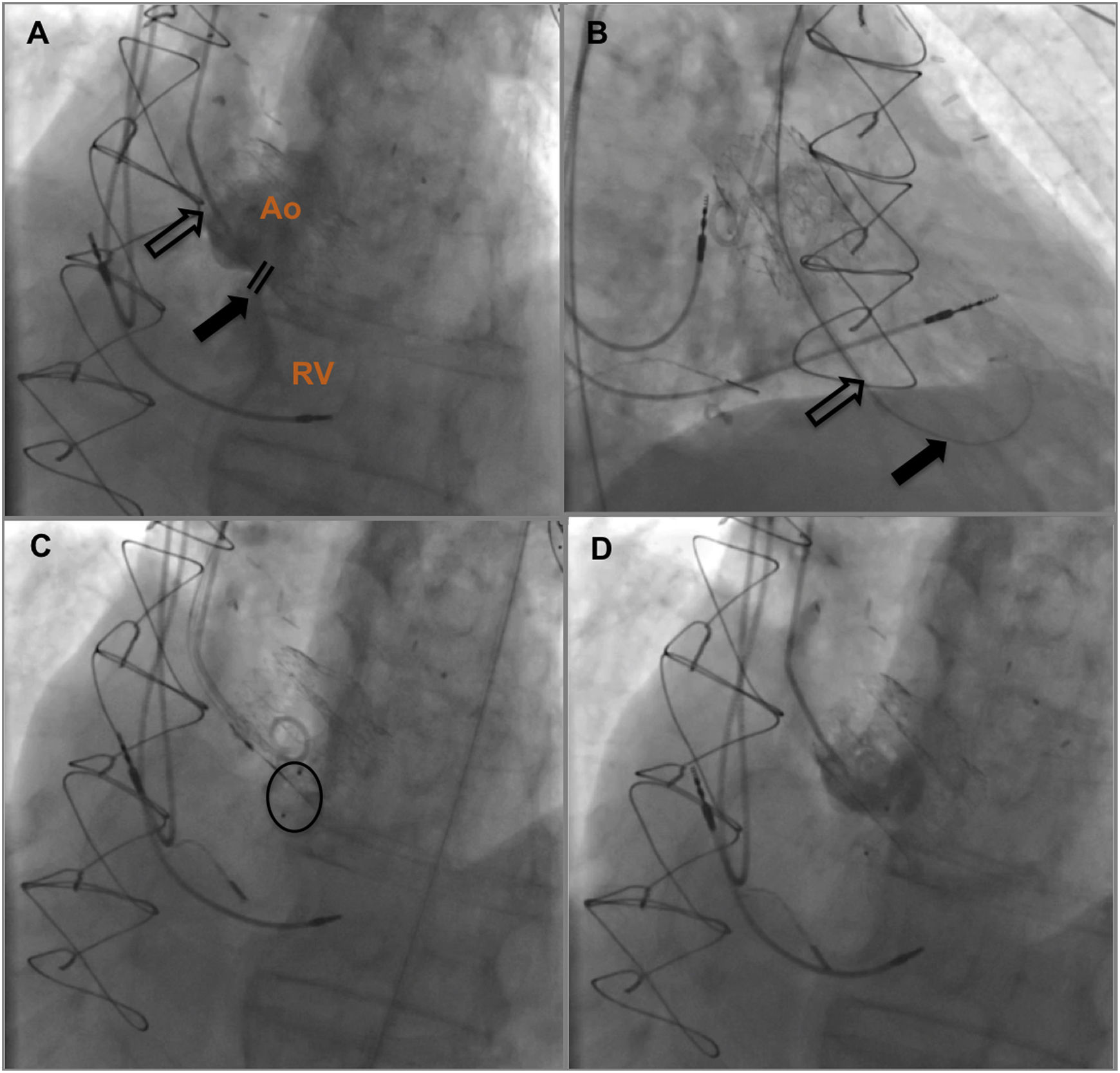
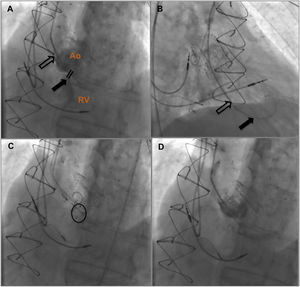
After discussion with the hospital's heart team and risk-benefit assessment, we decided that the surgical risk of reoperation was high and the patient was proposed for percutaneous closure. The procedure was performed under local anesthesia, with two arterial accesses (right radial 5F and femoral 6F) and one venous access for introduction of the ICE probe (right femoral vein, 8F). The ICE probe (ACUSON AcuNav ultrasound catheter, Siemens, Mountain View, CA) was placed in the right ventricle with optimal visualization and alignment with the fistula (Figure 2A). A 5F pigtail catheter was positioned in the ascending aorta, and the fistula's position was confirmed by angiography (Figure 3A). A 5F MP1 catheter was advanced through the right femoral artery, crossing the fistula into the right ventricle (Figures 2B and 3B). Through the MP1 an 0.035“Amplatzer guidewire was positioned in the right ventricle and the 5F diagnostic MP1 was exchanged for a 6F MP1 guiding catheter. The percutaneous closure device, a 6 mm Amplatzer Vascular Plug II (AVP) (Abbott, Chicago, IL), was then implanted (Figures 2C and 3C). ICE confirmed the correct positioning of the device, with minimal residual flow, and excluded interference with neighboring structures (Figures 2D and 3D). There were no complications regarding this procedure and the patient was discharged on the following day. At nine-month follow-up the patient is doing well, without heart failure or other complaints.
Fluoroscopic guidance of aorto-RV fistula closure. (A) Identification of aorto-RV fistula by aortography using a 5F pigtail catheter; (B) 6F MP1 catheter passing across the defect and an 0.035” Amplatzer guidewire positioned in the right ventricle; (C) 6 mm Amplatzer Vascular Plug II positioned; (D) after device deployment, no residual shunt was observed on angiography. Ao: aorta; RV: right ventricle.
Several mechanisms can lead to cardiac shunts after SAVR, including unintentional injury to the membranous septum sustained during dissection below the noncoronary cusp, perivalvular damage due to aggressive debridement of calcified areas of the aortic annulus, and ischemic necrosis due to inappropriate suturing of the prosthesis in the membranous septum.7
Recent minimally invasive techniques are increasingly challenging traditional approaches to aortic valve surgery. Rapid deployment and sutureless aortic valves are minimally invasive surgical options associated with short surgical duration, which may reduce postoperative mortality and morbidity and improve cost-effectiveness, particularly in high-risk patients as well as in those undergoing complex or concomitant procedures.8,9
The Perceval sutureless valve consists of a biological component of bovine pericardium fixed in a metal cage and has elastic properties that allow the stent to adapt to the anatomy of the aorta and to follow its movements, relieving the stress on the leaflets, which theoretically may reduce the risk of fistula formation.10 There has been only one published case of a fistula between the aorta and right chambers (right atrium) after a Perceval valve implantation, but in the context of previous bioprosthesis failure.11 In that case, the authors identified as potential mechanisms for fistulization local distortion of the aortic root because of the radial force from the Perceval valve auto-expanding mechanism, a fragile aortic root due to bicuspid aortic valve, and removal of the previous bioprosthesis with aggressive debridement. In our case, other contributing factors could be considered responsible for fistulization, such as concomitant myectomy or fixing the valve lower, at the level of the right sinus of Valsalva. Moreover, these valves may be associated with other complications similar to TAVI, with higher rates of permanent pacemaker implantation and paravalvular leakage (PVL) than conventional surgical valves. However, compared with TAVI, they are associated with lower early mortality and PVL. Stroke rates are also lower with the Perceval valve, but the difference is not statistically significant.8,10
Clinical presentationAorto-RV fistulae may have various clinical presentations depending on etiology, acute or chronic onset and size of shunt. Small leaks, with small shunt volume and limited cardiac overload, have a benign prognosis. However, larger fistulae may lead to congestive heart failure, which was the case of this patient; pulmonary hypertension and right ventricular failure is the ultimate expression, associated with poor outcome and high mortality.1 Hemolysis, with anemia and jaundice, may worsen the clinical course in these cases. Since the natural history of these fistulae after SAVR is poorly studied, careful follow-up of these patients is mandatory. No cases of spontaneous closure have been documented and aorto-RV shunts are in fact characterized by their propensity to increase in size over time.1 Hence, medical treatment, mainly based on diuretics, remains palliative and sometimes ineffective for relieving symptoms of heart failure in large fistulae.
TreatmentSurgery is the primary treatment of aorto-cardiac fistulae, although postoperative mortality after surgical correction can be as high as 54%.12 However, owing to major advances in interventional cardiology, percutaneous closure is now a safe and attractive alternative. Many devices have been developed, such as the Rashkind PDA occluder, the Amplatzer Duct Occluder, the Amplatzer ASD and PFO Occluder, various types of coils, and, more recently, the Amplatzer Plug.12,13 The AVP II is a redesigned version of the original vascular plug with finer, more densely woven nitinol wire as well as a three-segment design to favor rapid and complete occlusion. Compared with other devices, the Amplatzer Plug used in this case offers an easier way to occlude aorto-cardiac fistulae because the device can be deployed in a single-step procedure and does not require the placement of two parts on each side of the fistula. Because of its three-lobe design, the AVP II has six layers of mesh, giving the device better occlusive properties but also making it longer than the AVP.12,13 The three lobes can be shortened by compression, enabling better sealing and fitting into a short landing zone. It is recommended to select a plug diameter at least 20% larger than the target vessel.13
Echocardiographic guidance for percutaneous closureAlthough TEE imaging guidance (often with three-dimensional images) is well established and provides exceptional images, it most commonly requires general anesthesia and may be associated with intermittent obstruction of fluoroscopic viewing. ICE was already used in our center for guidance of other structural procedures, and in this case was an attractive alternative in a patient with high risk for general anesthesia due to hemodynamic instability. ICE has proved to be a safe technique for guiding percutaneous interventional treatments, with the advantage of requiring only local anesthesia and allowing reduction of radiation exposure, while maintaining high image resolution.14 However, from an economic point of view, possible savings from shorter procedural times (from avoiding general anesthesia and shorter hospital stays) need to be weighed against the cost of the ICE catheter, the need for specific operator skills and other potential complications like transient arrhythmias.15 Nonetheless, further studies comparing the accuracy, reproducibility, and outcomes of ICE guidance with guidance by TEE need to be performed prior to adopting ICE imaging as the primary non-radiographic imaging modality for structural heart disease procedures.
ConclusionAorto-RV fistula is an exceedingly rare finding after SAVR and to the best of our knowledge has never before been associated with a sutureless aortic valve replacement. Patient management depends strongly on clinical presentation and the presence of heart failure. Although surgical closure remains the gold standard to treat aorto-RV fistulae effectively, a percutaneous procedure with an appropriately selected device may be encouraged because of the high morbidity and mortality of redo open-heart surgery. To minimize the risk of a second general anesthesia, the use of ICE to guide the percutaneous procedure is feasible and safe, and grants high quality images.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
EthicsInformed consent has been obtained from the patient (or patient's guardian) for publication of the case report and accompanying images.
Conflicts of interestThe authors have no conflicts of interest to declare.