Tulip malformation is a newly defined complication of transcatheter atrial septal defect closure. This complication, in which the left atrial disc becomes concave, makes it impossible to fully retract the device into the delivery sheath. The case presented is the first report describing a simple new technique which overcomes this novel complication.
A malformação em tulipa é uma complicação recentemente descrita da oclusão percutânea da comunicação interauricular (CIA). Esta complicação em que o disco na aurícula esquerda adquire uma forma côncava durante o implante, impedindo a reintrodução do dispositivo na bainha. O caso apresentado é o primeiro relato que descreve uma forma nova e simples de superar este complicação.
Atrial septal defect (ASD) occluder device deformations are rare complications encountered during transcatheter ASD closure. Cobrahead device malformation is the most commonly reported of these complications.1 Recently, a new malformation, called tulip malformation, has been reported with the LifeTech Cera ASD occluder.2 Our case report is the second in the literature in which a tulip malformation was seen. However, a simple and previously unused technique was used to correct the deformation.
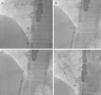
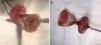
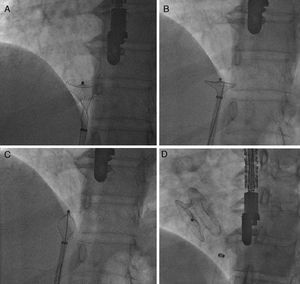
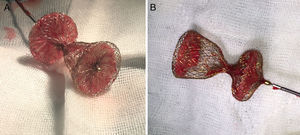
Case reportA 51-year-old woman diagnosed with secundum ASD was referred to our center for transcatheter closure. The presence of secundum ASD was confirmed by transesophageal echocardiography (TEE), which showed the patient's defect to be 19 mm in diameter. Under local anesthesia and under the guidance of TEE, a 12 F SteerEase delivery sheath (Lifetech Scientific, Shenzhen, China) was deployed in the left atrium (LA) over a 0.035” wire via the right femoral vein. A 22 mm Cera ASD device was then opened in the defect under fluoroscopic and TEE guidance. The attempt was unsuccessful because of failure of the device to approach the defect at the appropriate angle. During subsequent attempts, it was observed that the structure of the device had changed; the LA disc had become concave (Figure 1A) and could no longer be retracted into the sheath. This deformation could not be corrected by manipulations inside the LA. The device, which was partially retracted into the sheath, was then taken into the right atrium, which was safer. When further attempts in the right atrium failed, a different solution was considered. The stiff and flat side of a 0.038” J-wire was guided through the sheath containing the device. With the delivery cable in traction and held stable, an attempt was made to advance the hub of the LA disc with the stiff side of the 0.038” J-wire under fluoroscopy. After several attempts, the concave deformity was rectified by engaging the hub section, and the device was completely retracted into the sheath (Figure 1B and C, Video 1 and 2). The removed device was discovered to have a distorted structure, resembling a tulip (Figure 2). A new 26 mm Cera device was then successfully deployed in the defect (Figure 1D) and the procedure was completed.
Concave deformation of the distal left atrial (LA) disc preventing full device recapture (A); the stiff and flat side of a 0.038” J-wire guided through the sheath to the LA disc (B); concave deformity rectified by engaging the hub section, and the device completely retracted into the sheath (C); final position of the 26 mm Cera device after implantation (D).
The cobrahead malformation during transcatheter ASD closure has been thoroughly described.3,4 However, a tulip-shaped occluder deformation has only been reported once previously.2 Hayes et al. managed to rectify the concave deformation by capturing the hub of the LA disc with a snare through the right internal jugular vein. Given the fact that the hub of the LA disc is only 1–2 mm in diameter, this procedure can be said to be technically challenging and requires dexterity. It also requires separate vascular access. In our technique, neither separate vascular access nor extensive snare experience is required.
ConclusionTranscatheter closure of secundum ASD cases is a method that is frequently and successfully applied nowadays. It is inevitable that similar device malformations will be encountered in the future with increasing frequency of the procedure. Therefore, we believe that the new technique defined in our case will provide a relatively easy, effective and rapid solution to similar device malformations.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.