Infective endocarditis is a well-known clinical entity. However, despite improved diagnostic techniques and advances in treatment options, left-sided native valve infective endocarditis remains a serious disease with high morbidity and mortality, especially in cases caused by Staphylococcus aureus. The clinical heterogeneity of infective endocarditis sometimes prevents rapid recognition, correct diagnosis and timely treatment, which are essential to reduce the morbidity and mortality associated with this disease.
We report the case of a 62-year-old man, admitted for atrial fibrillation with complete atrioventricular block, which was found to be the result of methicillin-resistant S. aureus mitral valve endocarditis, complicated by local extension of the infection, heart failure, systemic embolism and multiple organ failure.
A endocardite infecciosa é uma entidade clínica profundamente estudada. Contudo, apesar da melhoria das técnicas de diagnóstico e dos avanços terapêuticos, a endocardite infecciosa de válvula nativa do coração esquerdo, continua a ser uma doença grave, com elevada morbilidade e mortalidade, principalmente nos casos de infeção por Staphylococcus aureus. A sua heterogeneidade clínica, por vezes, dificulta o rápido reconhecimento, correto diagnóstico e tratamento atempado, elementos fundamentais para a redução da morbi-mortalidade associada a esta doença.
Apresenta-se o caso de um homem de 62 anos, admitido por fibrilhação auricular com bloqueio aurículo-ventricular completo, que se constatou ser resultado de uma endocardite da válvula mitral, por St. aureus meticilino-resistente, complicada com extensão local da infecção, insuficiência cardíaca, embolização sistémica e disfunção multi-orgânica.
Bacterial endocarditis is a complex disease, associated with significant morbidity and mortality.
In developed countries, Staphylococcus aureus is now the leading cause of left-sided infective endocarditis, and is usually characterized by an acute presentation, without the classic physical findings. Its course is frequently fulminant when it involves the mitral or aortic valve, with widespread metastatic infection, and death in 25–30% of cases. For this reason, many patients present with advanced disease and multiple complications.
We present the case of a 62-year-old man admitted for atrial fibrillation with complete atrioventricular block, which was found to be the result of methicillin-resistant S. aureus mitral valve endocarditis, complicated by local extension of the infection, heart failure, systemic embolism and multiple organ failure.
Case reportA 62-year-old man presented to our hospital because of altered state of consciousness with profound asthenia for the previous two days. His medical history included hypertension, type 2 diabetes, obesity, hyperthyroidism, peptic ulcer disease and paroxysmal atrial fibrillation. He was taking amiodarone 200mg bid, warfarin, an angiotensin-converting enzyme inhibitor and oral hypoglycemic agents.
Physical examination on presentation revealed marked prostration, mild polypnea, apyrexia, normal blood pressure and a bradyarrhythmic pulse of 30bpm. Auscultation of heart and lungs revealed no major abnormalities. An electrocardiogram was performed and atrial fibrillation with complete atrioventricular block was detected. Significant laboratory results included normochromic normocytic anemia, relative neutrophilia, acute renal failure, mild hyperkalemia (5.3mEq/l), and elevated BNP (827pg/ml) and C-reactive protein (52mg/l). The bradyarrhythmia was considered to be related to amiodarone and a temporary pacemaker was implanted, without apparent immediate complications and with progressive clinical improvement.
Around six weeks before admission the patient had been seen in the emergency department after a car accident; he had had minor lesions and was discharged on NSAIDs. Three weeks later, he was admitted to the surgical department due to a bleeding upper gastrointestinal ulcer treated effectively with endoscopic therapy. During this hospitalization he was diagnosed with hyperthyroidism; he also had phlebitis in his right arm and methicillin-sensitive S. aureus (MSSA) bacteremia. The patient was advised to discontinue amiodarone because of hyperthyroidism, but he continued to take the drug.
One day after admission, he presented with fever and leukocytosis with additional elevation of inflammatory markers, and remained dependent on pacemaker rhythm. Blood cultures were collected and ceftriaxone was initiated. The next day, multiple separate blood cultures were positive for Gram-positive clustered cocci (eventually identified as methicillin-resistant S. aureus [MRSA]), and vancomycin was added.
On the fourth day, the site of pacemaker insertion, in the right femoral vein, presented a purulent drainage. As the patient remained pacemaker-dependent, the lead was removed and another was implanted on the left side.
Meanwhile, the patient presented an unfavorable evolution, with progressive sepsis, worsening renal function and refractory acute pulmonary edema. Invasive mechanical ventilation and renal replacement therapy were required and he was transferred to the intensive care unit.
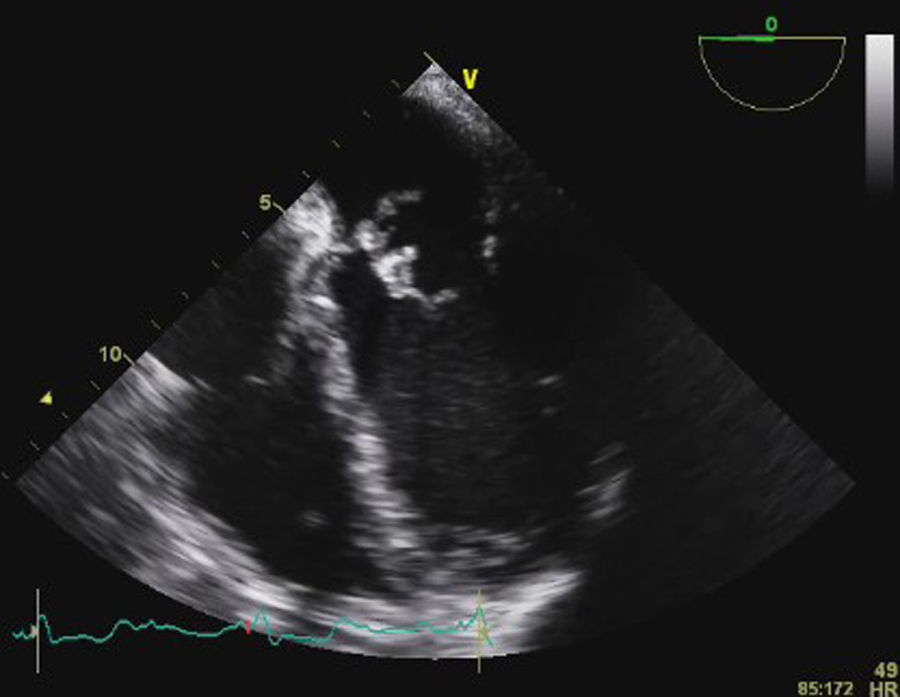
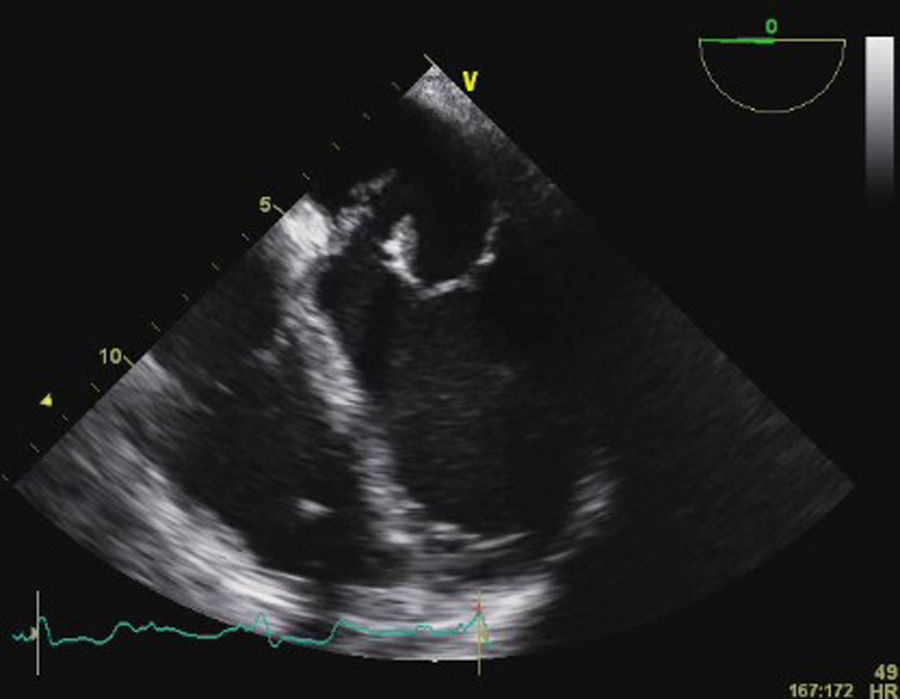
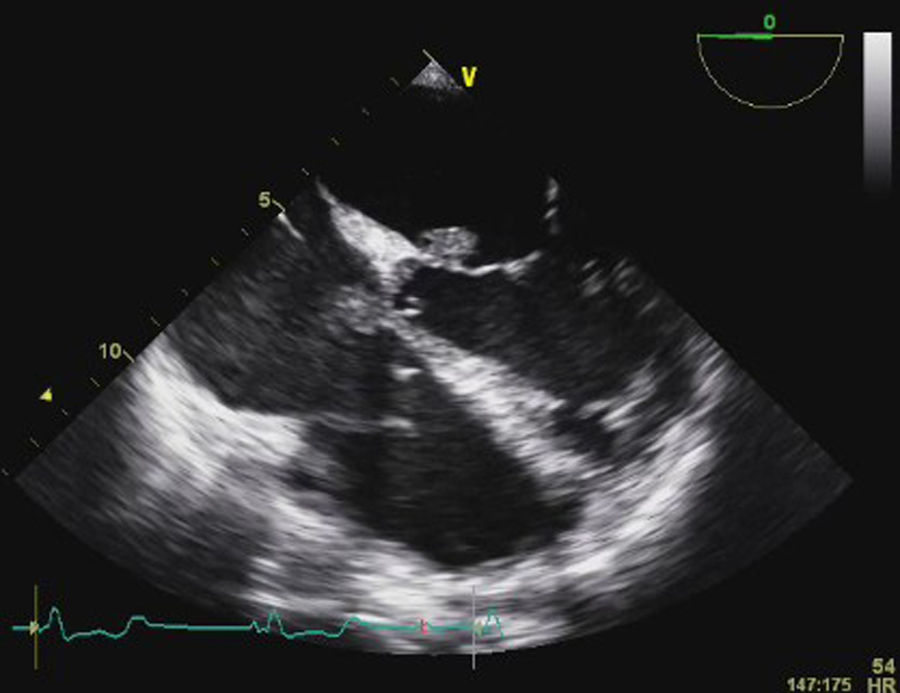
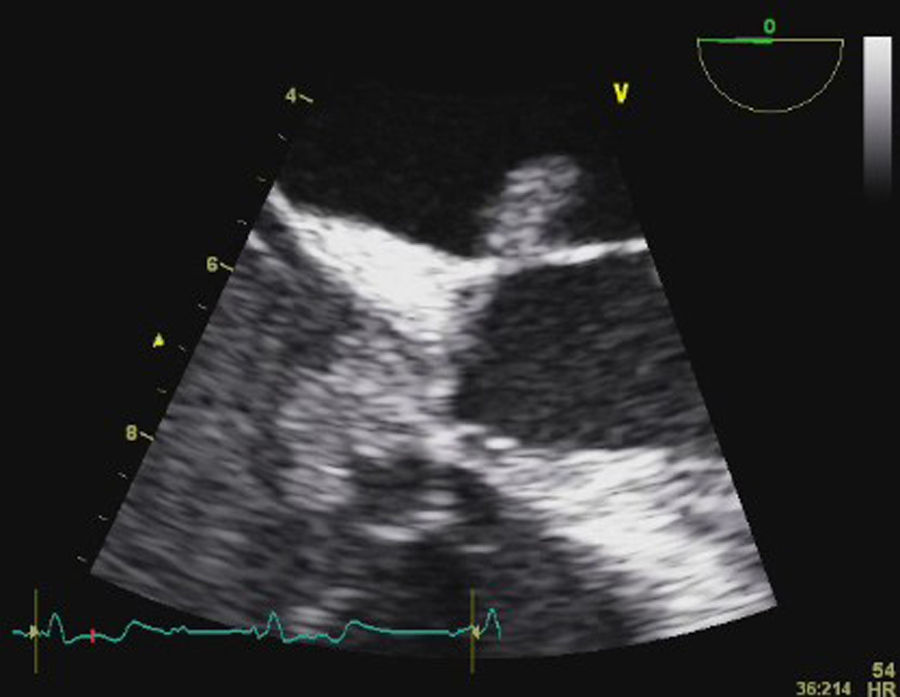
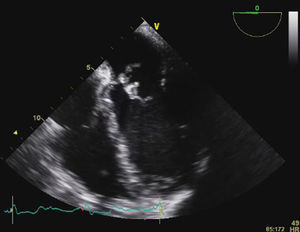
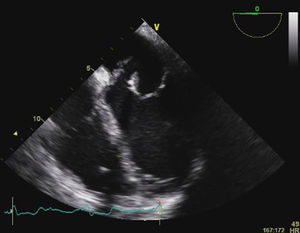
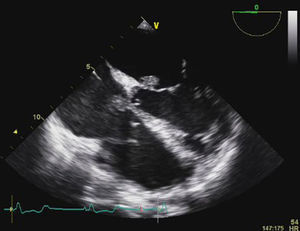
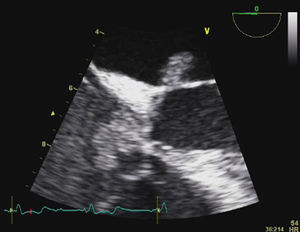
Transesophageal echocardiography (TEE) showed large vegetations on the atrial face of the anterior leaflet of the mitral valve (Figure 1; Video 1). The anterior mitral valve leaflet was perforated (Figure 2), causing severe mitral regurgitation (Video 2). Vegetations were also seen in the septal tricuspid leaflet, adjacent to the mitral valve (Figures 3 and 4; Videos 3 and 4).
Since the major affected valve was on the left side and the echocardiographic findings were so pronounced within a few days after pacemaker insertion, the endocarditis was considered to have resulted from the bacteremia detected in the previous hospitalization, with primary involvement of the mitral valve and extension of the infectious process to the mitral-aortic junction and tricuspid valve, with damage to the conduction system, causing the bradyarrhythmia that led to the present admission.
A cerebral CT scan was then performed that showed small low-density right-sided parieto-occipital and cerebellar lesions, suggestive of embolic infarction, without midline shift or signs of intracranial hemorrhage. Signs of splenic embolic infarctions were also found on the abdominal CT scan.
Although the patient had neurologic involvement, urgent surgery was considered given the presence of heart failure, locally uncontrolled infection and multiple embolic events (all major surgical indications), in the absence of cerebral hemorrhage. On day nine, the patient underwent mitral and tricuspid valve replacement surgery, with implantation of bioprosthetic valves. Post-operative TEE showed preserved biventricular systolic function, normally functioning bioprosthetic valves and no intracardiac shunts. Serial follow-up TEE showed no images of vegetations or abscesses.
Despite his favorable cardiac evolution, the patient presented a complicated course with multiple respiratory infections, and eventually died four months after admission.
DiscussionInfective endocarditis is a heterogeneous disease, associated with significant morbidity and mortality, despite improved diagnostic tools and expanded therapeutic options.1,2 Interestingly, neither its incidence nor mortality have declined in recent years.1
In developed countries, S. aureus is now the leading cause of left-sided infective endocarditis.1,3 The prevalence of endocarditis in patients with S. aureus bacteremia has been reported as 10–12%, and there is increasing agreement that echocardiography should be a routine procedure in these patients, in view of its frequency and the virulence of this organism and the devastating effects of infection.1,4
The clinical diagnosis of infective endocarditis requires a high index of suspicion because it may present as an acute, rapidly progressive infection, but also as a subacute or chronic disease. The symptoms are often only constitutional and many of the Oslerian manifestations are absent, except for subacute or chronic forms of the disease. Occasionally fever may be minimal or absent, especially in the elderly.1,5 Nevertheless, the diagnosis should be always considered in patients presenting with fever and a new regurgitant murmur, embolic phenomena, predisposing risk factors such as prosthetic devices, intravenous drug abuse and immunosuppression, or bacteriemia.1,2,5,6
S. aureus endocarditis is usually characterized by an acute presentation, without the classic physical findings. Its course is frequently fulminant when it involves the mitral or aortic valve, with widespread metastatic infection, and death in approximately 25–30% of cases.2 For this reason, many patients present with advanced disease and multiple complications, as in the case of our patient, who demonstrated the typical presentation of S. aureus endocarditis, with a locally destructive infection, heart failure, systemic embolism, sepsis and multiple organ dysfunction.
Embolic phenomena occur in 20–50% of cases and are frequently the first presentation of infective endocarditis.1 The risk of embolic events diminishes dramatically after initiation of antimicrobial therapy, particularly after the second week of treatment.1,2,6 The incidence of embolism is higher when there is involvement of left-side valves, especially the mitral valve (particularly the anterior leaflet), in the case of large (>10mm) and mobile vegetations, and when the causative agent is S. aureus, fungus or Streptococcus bovis.1,2,5,6 The brain and spleen are the most frequent sites of embolism of left-sided infective endocarditis, while pulmonary embolism is common in native right-sided and pacemaker lead infections.1,2 The occurrence of embolic events confers a worse prognosis, particularly if associated with a neurologic event.1,6
Although clinical signs are extremely important, the cornerstones of infective endocarditis diagnosis are blood cultures and echocardiography, which are the major criteria of the Duke classification.1,2,5,6 According to these criteria our patient had definite endocarditis: positive blood cultures, compatible echocardiographic findings (vegetation, leaflet perforation), fever, predisposing factors and multiple embolic events.
Treatment of infective endocarditis consists of eradication of infectious foci, antimicrobial therapy and, when indicated, early surgical therapy.
Antiplatelet and antithrombotic therapies are controversial. There is no strong evidence for the initiation of antithrombotic drugs during the active phase of infective endocarditis, in order to reduce embolic events, and some studies show a non-significant increase in major bleeding episodes. In patients already taking oral anticoagulants, there is a risk of intracranial hemorrhage, highest in patients with S. aureus prosthetic valve endocarditis and in those with a previous neurologic event, and therefore the decision for its continuation should be individualized.1
There are various antimicrobial regimens according to the microbial agent, its drug susceptibility profile and patient characteristics.1,6 The optimal antibiotic regimen for MRSA native-valve infection is a course of 4–6 weeks of vancomycin. The efficacy of aminoglycosides in these circumstances is not demonstrated and so the use of gentamicin for the first 3–5 days is optional.1
Surgical therapy in the active phase of the disease should be considered in the presence of heart failure or uncontrolled infection and to prevent embolic events.1 Our patient had major indications for early surgery: severe mitral regurgitation and recurrent acute pulmonary edema, perivalvular extension of the infection and leaflet perforation, and the presence of large vegetations with more than one embolic event. However, while timely surgical therapy substantially reduces early and late mortality, the risk of neurological deterioration with surgery after a neurologic event is not negligible. In these situations, the decision to operate should be discussed with a multidisciplinary group and should be individualized. Even so, according to recent studies, after a ischemic neurologic event, if cerebral hemorrhage has been excluded and neurological damage is not severe, surgery, if indicated, should not be delayed and can be performed with a relatively low neurological risk (3–6%).1 Our patient had small lesions, compatible with brain infarctions, without mass effect or hemorrhage, and major indications for surgery, so it was decided to proceed to surgical treatment.
The in-hospital mortality of infective endocarditis ranges from 10 to 26%. Heart failure, periannular complications and S. aureus infections are the most powerful predictors of a poor in-hospital outcome.1 Prognosis is much better in the case of right-sided endocarditis, especially in intravenous drug users (mortality <5%).2 Long-term prognosis is highly variable and is mainly dependent on age, presence of heart failure and comorbidities.1
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.