High-density lipoprotein cholesterol (HDL-C) is known to be a key player in reverse cholesterol transport and this function is associated with its atheroprotective role. Low HDL-C is a strong independent risk factor for cardiovascular disease, premature atherosclerosis and increased oxidative stress. However, the clinical benefit of high HDL-C through diet or drug therapy is still controversial.
MethodsThis study included 50 patients with isolated low HDL-C (≤35 mg/dl), 52 patients with isolated high HDL-C (≥70 mg/dl), and 33 age- and gender-matched controls with normal HDL-C. ‘Isolated’ was defined as excluding all clinical conditions associated with increased oxidative stress and inflammation, which may affect the structural state of HDL-C. In addition to arylesterase (ARES) activity and plasma thiol levels, laboratory parameters associated with oxidative stress were also assessed.
ResultsLevels of ARES (758 [169-1150] vs. 945 [480-1215] and 821 U/l [266-1220]; p<0.01) and total thiols (233±41 vs. 259±46 μmol/l; p=0.02) were markedly higher in patients with high HDL-C. More importantly, total antioxidant capacity, oxidative stress index, creatine and serum ARES levels were associated with changes in serum HDL-C levels.
ConclusionIn patients with isolated high HDL-C, we determined that while serum ARES activity and plasma thiol concentrations were significantly higher, the other markers associated with oxidative stress decreased markedly. Additionally, the present study demonstrated that serum oxidative stress status is very important to maintain the positive effects of HDL-C.
O colesterol de lipoproteínas de alta densidade (C-HDL) e o seu papel no transporte reverso de colesterol estão associados a um papel atero-protetor. O C-HDL baixo é um potente fator de risco independente para a doença cardiovascular (CV), para aterosclerose prematura e para o aumento do stress oxidativo. No entanto, o benefício clínico do aumento do colesterol HDL elevado através de dieta ou de terapêutica medicamentosa ainda é controverso.
MétodosEste estudo incluiu 50 doentes com C-HDL baixo «isolado» (≤35 mg/dL), 52 doentes com C-HDL elevado «isolado» (≥70 mg/dL) e 33 doentes controlo com C-HDL normal, emparelhados por idade e sexo. O termo «isolado» foi definido por exclusão de todas as condições clínicas associadas ao aumento do stress oxidativo e da inflamação que podem afetar o estado estrutural do C-HDL. Para além da atividade da arilesterase e dos níveis de tiol plasmático, foram também avaliados parâmetros laboratoriais associados ao stress oxidativo.
ResultadosOs níveis de arilesterase [758 (169-1150) versus 945 (480-1215); 821 (266-1220); p<0,01] e o total de tióis [233±41 versus 259±46; p=0,02] estavam significativamente mais elevados nos doentes com C-HDL elevado. Mais relevante, os níveis de TAC, OSI, creatina e ARES sérica associaram-se a alterações nos níveis séricos de C-HDL.
ConclusãoEm doentes com C-HDL isolado elevado demonstrámos que a atividade sérica de ARES e as concentrações de tiol plasmático se encontram significativamente elevadas, enquanto os outros marcadores associados ao stress oxidativo estão significativamente diminuídos. Além disso, o presente estudo demonstrou que o estado de stress oxidativo sérico é muito importante para manter os efeitos positivos do C-HDL.
High-density lipoprotein cholesterol (HDL-C) is a plasma lipoprotein that is heterogeneous in terms of origin, size, composition, and function. It is known to be a key player in reverse cholesterol transport, a function associated with its atheroprotective role. Its other, more prominent atheroprotective roles include its anti-inflammatory, antioxidant, antiapoptotic, and antithrombotic functions.1–3
Oxidative stress is defined as an excess production of reactive oxygen species (ROS) relative to the levels of antioxidants, creating an imbalance between pro-oxidant and antioxidant factors. ROS play an important role in the pathogenesis of atherosclerosis.4,5 Most evidence suggests that serum HDL-C has various antioxidant properties, such as reducing the harmful effects of ROS production, increasing endothelial NO synthase activity, and preventing low-density lipoprotein cholesterol (LDL-C) oxidation.6–8 These beneficial effects are associated with enzymes such as paraoxonase-1 (PON-1) on the surface of HDL. Arylesterase (ARES) activity, predominantly conferred by HDL-associated PON-1, is a typical functional measure reflective of HDL-associated antioxidant activity.9–11
Epidemiological studies have demonstrated that low HDL-C is a strong independent risk factor for cardiovascular (CV) disease, premature atherosclerosis, and increased oxidative stress.12–15 However, the clinical benefit of high HDL-C levels through diet or drug therapy is still controversial.16 The disappointing results of studies on high HDL-C have often been associated with unfavorable variations in HDL-C function associated with increased serum oxidative stress status.17,18 Accordingly, we aimed to investigate the favorable effects of isolated serum HDL-C levels in terms of serum oxidative stress.
MethodsPatientsThe study population consisted of 50 patients with isolated low HDL-C (≤35 mg/dl), of whom 25 were female and 25 male, with mean age 56±14 years (low HDL-C group); 52 patients with isolated high HDL-C (≥70 mg/dl), 25 female and 27 male, with mean age 54±16 years (high HDL-C group); and a control group consisting of 33 age-, gender-, and body mass index-matched individuals with normal HDL-C (>35 mg/dl) (17 female and 16 male; mean age 58.6±13.8 years). The three groups were selected consecutively from the eligible patients during the same study. For the present study, ‘isolated’ was defined as excluding all clinical conditions associated with increased oxidative stress and inflammation, which may affect the structural state of HDL-C. Patients in all three groups were newly diagnosed and were not using any drugs affecting HDL-C levels or oxidative or inflammatory status. To minimize the influence of non-HDL-C parameters on lipid metabolism, the three groups were matched according to plasma triglyceride and LDL-C levels, which were calculated using the Friedewald formula. Exclusion criteria were coronary heart disease (CHD), valvular heart disease, hypertension, heart failure, peripheral arterial disease, diabetes, renal or hepatic dysfunction, hematological disorders, history of malignancy, history of psychiatric disease, acute or chronic infection, obesity (defined as body mass index values over the 95th percentile for age and gender), smoking, alcohol use, and any drug use affecting lipids. Informed consent was obtained from all patients, and the study was approved by the institutional ethics committee.
Blood sample collectionBlood samples were drawn from an antecubital vein by careful venipuncture using a 21G needle without stasis between 8.00 a.m. and 10.00 a.m. after a fasting period of 12 hours. The collected samples were centrifuged at maximum relative centrifugal force of 3000×g for 10 min and the serum was separated. Glucose, creatinine, aspartate aminotransferase, alanine aminotransferase, electrolytes, lipid profiles, and thyroid stimulating hormone were determined by standard methods on the same day and the samples were stored at -80°C until analysis for measurement of total antioxidant capacity (TAC), total oxidative status (TOS), and total thiol and ARES levels. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation. Hematological indices were measured within 30 min of the blood samples being placed in tubes containing dipotassium EDTA. Biochemical analyses were performed using an Olympus AU-640 autoanalyzer (Olympus Diagnostics Hamburg, Germany). An automated blood counter (Beckman-Coulter Co, Miami, FL) was used for whole blood counts.
Measurement of thiol levelsTotal serum thiol levels were measured using the novel automated spectrophotometric method described by Erel and Neselioglu.19 In this method, disulfide bonds were first reduced to form free functional thiol groups with sodium borohydride. Remnant reductant sodium borohydride was consumed and removed with formaldehyde to prevent reduction of DTNB [5,5’-dithiobis-(2-nitrobenzoic) acid], and all of the thiol groups, including reduced and native thiol groups, were determined spectrophotometrically after the reaction with DTNB at 412 nm. Total thiol levels were then calculated and the results are presented as μmol/l.19
Measurement of arylesterase levelsSerum ARES activity was assessed using phenyl acetate as a substrate, measured by UV spectrophotometry in a 96-well plate format (Spectramax 384 Plus, Molecular Devices, Sunnyvale, CA). Enzyme activity was calculated from the molar absorptivity coefficient of the produced phenol, 1310 M-1 cm-1. One unit of ARES activity was defined as 1 μmol phenol generated/min under the above conditions and expressed as U/l serum.20
Measurement of total antioxidant capacity and total oxidative statusTAC and TOS were examined in every patient group. Blood samples were obtained following overnight fasting. For TAC/TOS measurements, 5 ml of blood was collected into a plastic tube containing potassium EDTA. The serum was separated from the cells by centrifugation for 10 min and then stored at -80°C until biochemical examination. TAC and TOS levels were measured using commercially available kits (Rel Assay, Turkey). TAC level was measured using a novel automated method, based on the bleaching of the characteristic color of a more stable 2.20-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation by antioxidants. The results were expressed as mmol Trolox Eq/l. TOS level was measured by a method in which oxidants present in the sample oxidize the ferrous-ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules abundantly present in the reaction medium. The ferric ion produces a colored complex with xylenol orange in an acidic medium. Color intensity, which is measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide, and the results were expressed in terms of micromolar hydrogen peroxide equivalent/l (mmol H2O2 Eq/l).21 The ratio of TOS to TAC was accepted as the oxidative stress index (OSI). For calculation, the resulting unit of TAC was converted to mmol/l, and the OSI value was calculated according to the following formula: OSI (arbitrary unit) 1/4 TOS (mmol H2O2 Eq/l)/TAC (mmol Trolox Eq/l).22
Statistical analysisData were analyzed with IBM SPSS version 23.0 for Windows. Distribution of continuous variables was tested by the Kolmogorov-Smirnov test. Continuous variables were expressed as mean ± standard deviation or median and 25th-75th percentile (interquartile range [IQR]) for normal and non-normal distributions, respectively. Categorical variables were expressed as percentages. Statistical differences between groups were tested by one-way analysis of variance with the post-hoc Scheffé test or the Kruskal-Wallis test for parametric or nonparametric variables, respectively. Categorical variables were compared using the chi-square test. Spearman's or Pearson's correlation coefficients were calculated to assess relationships between variables. A univariate linear regression model was used to adjust differences in HDL-C levels between groups for age and gender. Thereafter, linear regression analyses were performed stepwise to identify the possible association of HDL-C levels as a dependent variable with potential confounding factors among the three groups. These confounders were creatinine, uric acid, eGFR, OSI, TOS, TAC, total thiol and ARES levels. A two-tailed p<0.05 was considered significant.
ResultsBaseline characteristics of the three groups are presented in Table 1. Age and gender were comparable between the groups, as were systolic and diastolic blood pressure, heart rate, and waist circumference. As expected, body mass index was higher in the low and high HDL-C groups than the control group (p<0.01). Routine biochemical tests were generally comparable between the groups, with the exception of alanine transaminase (p<0.01) (Table 2). Additionally, eGFR was lower and creatinine levels were higher in the low HDL-C group than in the high HDL-C and control groups (p<0.01). Total cholesterol levels were significantly higher in the high HDL-C group than the other groups (p<0.01). Triglyceride levels were higher in the low HDL-C group than the high HDL-C group (p<0.01). Non-HDL-C values were markedly higher in the low HDL-C group than the high HDL-C group (p=0.02). LDL-C values were comparable between groups (p=0.39).
Demographic and clinical characteristics of the three study groups.
| Low HDL-C group (n=50) | High HDL-C group (n=52) | Control group (n=33) | p | |
|---|---|---|---|---|
| Mean age, years | 56±14 | 54±16 | 58±13 | 0.52 |
| Male/female | 25/25 | 25/27 | 16/17 | 0.98 |
| BMI, kg/m2 | 26.7±2.4* | 26.8±2.4* | 24.6±3.1 | <0.01 |
| Waist circumference, cm | 101±10 | 101±12 | 96±11 | 0.10 |
| Systolic BP, mmHg | 124±17 | 128±17 | 119±26 | 0.10 |
| Diastolic BP, mmHg | 79±10 | 78±10 | 78±13 | 0.65 |
| Heart rate, bpm | 79±12 | 79±9 | 76±14 | 0.45 |
BP: blood pressure, BMI: body mass index; HDL-C: high-density lipoprotein cholesterol.
Laboratory characteristics of the three study groups.
| Low HDL-C group (n=50) | High HDL-C group (n=52) | Control group (n=33) | p | |
|---|---|---|---|---|
| Glucose, mg/dl | 98±13 | 97±11 | 93±21 | 0.32 |
| Creatinine, mg/dl | 0.98±0.17* | 0.85±0.14 | 0.85±0.12 | <0.01 |
| eGFR, ml/min/1.73 m2 | 76±12** | 89±17 | 80±15 | <0.01 |
| Sodium, mg/l | 139±2.6 | 140±2.2 | 139±5.4 | 0.51 |
| Potassium, mg/l | 4.3±0.4 | 4.4±0.4 | 4.2±0.4 | 0.19 |
| AST, U/l | 21±13 | 19±3 | 17±5 | 0.11 |
| ALT, U/l | 27±17* | 19±6 | 22±4 | <0.01 |
| TSH, mIU/l | 1.80 (0.3-5.7) | 2.0 (0.2-5) | 1.53 (0.1-4.8) | 0.22 |
| Hemoglobin, g/dl | 14.0±1.19 | 13.6±1.06 | 14.1±1.58 | 0.27 |
| Platelets, 103/mm3 | 260±113 | 256±51 | 240±66 | 0.52 |
| TC, mg/dl | 167±24 | 196±36* | 176±28 | <0.01 |
| LDL-C, mg/dl | 107±26 | 100±17 | 107±23 | 0.39 |
| Triglycerides, mg/dl | 138±43** | 115±25 | 117±43 | <0.01 |
| HDL-C, mg/dl | 30±2.4* | 81±14* | 46±6 | <0.01 |
| Non-HDL-C, mg/dl | 136±24** | 119±36 | 130±27 | 0.02 |
ALT: alanine transaminase, AST: aspartate transaminase, eGFR: estimated glomerular filtration rate, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, TC: total cholesterol, TSH: thyroid stimulating hormone.
Oxidative stress parameters in the three study groups are shown in Table 3. There were significant differences in parameters associated with oxidative stress among the three groups. TOS levels (48.3 μmol H2O2 Eq/l [range 7.8-111] vs. 15.3 μmol H2O2 Eq/l [range 1.9-92.9] vs. 35.2 μmol H2O2 Eq/l [range 5.1-64.0], respectively; p<0.01), TAC levels (1.37 mmol Trolox Eq/l [range 1.07-1.57] vs. 1.24 mmol Trolox Eq/l [range 0.76-1.54], respectively; p<0.01) and the OSI ratio (35.7 [range 6.72-87.2] vs. 12.3 [range 1.54-67.8] vs. 26.9 [range 3.55-53.9], respectively; p<0.01) were significantly lower in the high HDL-C group than in the low HDL-C and control groups. Gamma-glutamyltransferase (35.1 U/l [range 10-122] vs. 20.9 U/l [range 11-72] vs. 21.8 [11-76]; p<0.01) and uric acid levels (6.3 U/l [range 3.3-8.9] vs. 5.1 U/l [range 2.8-8.3]; 4.4 U/l [range 2.8-8.2], respectively; p<0.01) were markedly increased in the low HDL-C group. Total thiol levels were significantly higher in the high than in the low HDL-C group (233±41 vs. 259±46 μmol/l, respectively; p=0.02). Serum ARES levels were higher in the high HDL-C group than in the other groups (945 U/l [range 480-1215] vs. 758 U/l [range 169-1150] and 821 U/l [range 266-1220], respectively; p<0.01) (Table 3).
Comparison of parameters associated with oxidative stress in the three study groups.
| Low HDL-C group (n=50) | High HDL-C group (n=52) | Control group (n=33) | p | |
|---|---|---|---|---|
| TOS, μmol H2O2 Eq/l | 48.3 (7.8-111) | 15.3 (1.9-92.9)* | 35.2 (5.1-64.0) | <0.01 |
| TAC, mmol Trolox Eq/l | 1.37 (1.07-1.57) | 1.24 (0.76-1.54)* | 1.31 (0.77-1.54) | <0.01 |
| OSI, AU | 35.7 (6.72-87.2) | 12.3(1.54-67.8)* | 26.9 (3.55-53.9) | <0.01 |
| ALP, U/l | 72±27 | 72±16 | 71±16 | 0.97 |
| GGT, U/l | 35.1 (10-122)* | 20.9 (11-72) | 21.8 (11-76) | <0.01 |
| Uric acid, mg/dl | 6.3 (3.3-8.9)* | 5.1 (2.8-8.3) | 4.4 (2.8-8.2) | <0.01 |
| ARES, U/l | 758 (169-1150) | 945 (480-1215)* | 821 (266-1220) | <0.01 |
| Total thiols, μmol/l | 233±41** | 259±46 | 245±53 | 0.02 |
ALP: alkaline phosphatase; ARES: arylesterase; AU: arbitrary units; GGT: gamma-glutamyltransferase; HDL-C: high-density lipoprotein cholesterol, OSI: oxidative stress index; TAC: total antioxidant capacity; TOS: total oxidative status.
In the correlation analysis, HDL-C levels were moderately negatively correlated with TAC (p<0.001, r=0.378), TOS (p<0.001, r=0.513), OSI (p<0.001, r=0.489) and creatinine levels (p<0.001, r=0.365). However, they were moderately positively correlated with serum ARES activity (p<0.001, r=0.40) and eGFR (p<0.001, r=0.351).
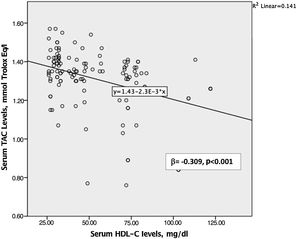
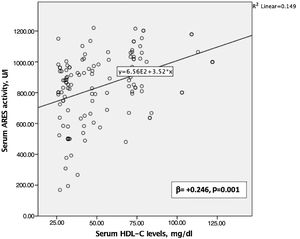
Creatinine, uric acid, eGFR, OSI, TOS, TAC, and total thiol and serum ARES levels differed among the groups and were entered into a stepwise linear regression analysis. A multivariate analysis was then performed between them to identify independent predictors associated with HDL-C levels. Among the univariate predictors, TAC levels (β=-0.309, 95% confidence interval [CI]: -78.471 to 28.734, p<0.001, Figure 1), OSI levels (β=-0.383, 95% CI: -0.636 to 0.282, p=0.001) and ARES levels (β=+0.246, 95% CI: 0.11 to 0.43, p=0.001, Figure 2) were dependently associated with HDL-C values (Table 4).
Linear regression analysis of the effect of total antioxidant capacity, oxidative stress index and arylesterase levels on plasma high-density lipoprotein cholesterol levels as a dependent variable.
| Model | Unstandardized coefficients (B) | Standardized coefficients (beta) | 95% CI for B | p |
|---|---|---|---|---|
| (constant) | 112.043 | 74.280 to 149.806 | 0.000 | |
| OSI | -0.459 | -0.383 | -0.636 to 0.282 | 0.000 |
| TAC | -53.603 | -0.309 | -78.471 to 28.734 | 0.000 |
| ARES | 0.027 | 0.246 | 0.11 to 0.43 | 0.001 |
ARES: arylesterase; CI: confidence interval; OSI: oxidative stress index; TAC: total antioxidant capacity.
To the best of our knowledge, the present study is the first to assess oxidative stress parameters in patients with ‘isolated’ high HDL-C and low HDL-C. ARES and total thiol levels were markedly higher in patients with high HDL-C compared with low HDL-C subjects. Similarly, other markers associated with oxidative stress, such as TAC, TOS, OSI, uric acid and GGT, were also significantly elevated in the low HDL-C group. More importantly, TAC, OSI and serum ARES levels were associated with changes in HDL-C levels. Furthermore, serum creatine levels and glomerular filtration rate were markedly higher in the high HDL-C group.
The low HDL-C state, the most common form of dyslipidemia in CHD, is an independent risk factor for CV disease, premature atherosclerosis and increased oxidative stress.12–15
Post-hoc analysis from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial showed that low HDL-C levels were associated with increased CV risk in patients with stable ischemic heart disease, despite optimal medical therapy. Furthermore, this effect persisted even after LDL-C levels returned to a normal range.23 In another important study, medically treated high-risk acute coronary syndrome patients with low baseline HDL-C levels (<30 mg/dl) had a higher risk of long-term CV and all-cause death. A similar risk was found for nonfatal ischemic outcomes in patients with high baseline HDL-C levels (≥60 mg/dl).24 However, the emerging evidence associated with serum HDL-C levels increased by drug therapy is still conflicting.16 It has been suggested that the disappointing results in patients with high serum HDL-C levels are associated with dysfunctional or proatherogenic forms of HDL particles caused by increased inflammation and oxidative stress. Patients with coronary artery disease, chronic kidney disease and rheumatoid arthritis showed multiple alterations in the protein composition of HDL. These studies revealed phospholipid depletion and enrichment with proinflammatory and oxidative proteins in HDL particles. Additionally, cholesterol efflux from lipid-laden macrophages by HDL-C has been claimed to be less effective in inflammatory diseases.25–28 All of these important studies highlighted the unfavorable effects of increased serum oxidative stress and inflammation status on HDL-C. Hence, in the present study, we investigated oxidant and antioxidant functions of isolated HDL-C in terms of serum oxidative stress and inflammatory status.
Serum HDL-C has various antioxidant properties, such as reducing the harmful effects of ROS production, increasing endothelial nitric oxide synthase activity and preventing LDL oxidation.6–8 ARES activity, mainly conferred by HDL-associated PON-1, reflects the antioxidant activity of HDL-C. This activity is responsible for the hydrolysis of organophosphates to protect HDL-C and LDL-C from oxidation.9–11 Studies have demonstrated that serum ARES activity and HDL-C levels are significantly lower in diabetic patients with heart failure. Similarly, our study detected a moderate positive correlation between serum ARES activity and HDL-C levels and suggested that impaired antioxidant activity of HDL may be associated with the development of heart failure.29 Tang et al. found that serum HDL-C and ARES levels decreased significantly in cases of heart failure and that serum ARES activity was positively associated with HDL-C levels.30 Tabur et al. found that HDL-C levels were significantly lower in non-diabetic metabolic syndrome patients and non-metabolic syndrome obese patients. In contrast to our results, serum ARES levels were found to be comparable in the metabolic syndrome group, while TAC levels were lower.31 In other study with similar findings to ours, serum HDL-C levels in metabolic syndrome patients were significantly lower, although serum ARES activity, TAC, TOS and OSI levels were significantly higher than in the control group.32 Similarly to our study, Cervellati et al., demonstrated that serum ARES activity was higher in the high than in the low HDL-C group and negatively correlated with the presence of arterial plaques.33
Low molecular weight thiols and disulfides are critical components of the extracellular antioxidant defense system and play a significant role in the defense against ROS.34,35 Studies of hyperlipidemic patients have demonstrated a positive correlation between serum HDL-C and plasma thiol concentrations.36,37 Similarly, in the present study, total thiol levels were lower in the low HDL-C group and slightly positively correlated with serum HDL-C levels. Another study suggested decreased ARES activity attributed to increased oxidation of plasma protein thiols.38,39 In the present study, we did not detect a relationship between serum ARES activity and total thiol levels.
Several limitations of this study should be borne in mind. First, the number of patients was small because we used very strict exclusion criteria. As expected, this limited the statistical power of the study. Second, our analysis was based on a simple baseline determination at a single time point that may not reflect the patient's status over long periods. Although we excluded all conditions that interact with inflammation and oxidative stress, we were able to measure the amount of dysfunctional HDL-C or proatherogenic forms of HDL-C particles instead of measuring serum HDL-C.
ConclusionIn patients with isolated high HDL-C, we determined that while serum ARES activity and plasma thiol concentrations were significantly higher, the other markers associated with oxidative stress decreased markedly. Additionally, the present study demonstrated that serum oxidative stress status is very important to maintain the positive effects of HDL-C. Hence, we suggest that serum oxidative stress levels be improved via diet, exercise or medication, which may be useful in terms of HDL-C function.
Conflicts of interestThe authors have no conflicts of interest to declare.