We studied genotypic and allelic frequencies of polymorphisms that can affect platelet function, namely the Kozak, VNTR and HPA-2 polymorphisms of glycoprotein Ibα, the PlA polymorphism of glycoprotein IIIa and the C807T polymorphism of glycoprotein Ia, in a Portuguese population composed of 227 donors.
MethodsPCR-RFLP was used to assess the Kozak, HPA-2, PlA and C807T polymorphisms. The VNTR polymorphism was discriminated by different weight bands on electrophoresis.
ResultsAll genotypic frequencies were in Hardy-Weinberg equilibrium and do not differ from other Caucasian populations. Genotypic frequencies were 68.3%, 26.9% and 4.8% for PlA1/A1, PlA1/A2 and PlA2/A2 genotypes of the PlA polymorphism, 79.3%, 20.3% and 0.4% for TT, TC and CC genotypes of the Kozak polymorphism, 81.1%, 18.9% and 0.0% for aa, ab and bb genotypes of the HPA-2 polymorphism, 15.4%, 0.9%, 70.5%, 11.5%, 1.3% and 0.4% for BC, BD, CC, CD, DD and CE genotypes of the VNTR polymorphism, and 39.7%, 50.2% and 10.1% for CC, CT and TT genotypes of the C807T polymorphism.
ConclusionsThe Portuguese population has now been characterized in terms of major platelet glycoprotein polymorphisms, which will be an important tool for further studies to assess the role of platelet glycoproteins in individual predisposition to prothrombotic conditions and response to antithrombotic therapy.
Neste estudo determinámos as frequências alélicas e genotípicas de 5 polimorfismos que poderão afetar a funcionalidade plaquetária, nomeadamente os polimorfismos Kozak, VNTR e HPA-2 da glicoproteína Ibα, o polimorfismo PlA da glicoproteína IIIa e o polimorfismo C807T da glicoproteína Ia numa população portuguesa constituída por 227 dadores voluntários.
MétodosA técnica de PCR-RFLP foi usada para determinar os polimorfismos Kozak, HPA-2, PlA e C807T. O polimorfismo VNTR foi discriminado através do padrão eletroforético criado por bandas de peso molecular diferente.
ResultadosAs frequências genotípicas encontram-se dentro do equilíbrio de Hardy-Weinberg e não diferem estatisticamente de outras populações caucasianas. As frequências genotípicas encontradas foram 68,3, 26,9 e 4,8% para os genótipos PlA1/A1, PlA1/A2 e PlA2/A2 do polimorfismo PlA, 79,3, 20,3 e 0,4% para os genótipos TT, TC e CC do polimorfismo Kozak, 81,1, 18,9 e 0,0% para os genótipos aa, ab e bb do polimorfismo HPA-2, 15,4, 0,9, 70,5, 11,5, 1,3 e 0,4% para os genótipos BC, BD, CC, CD, DD e CE do polimorfismo VNTR, e 39,7, 50,2 e 10,1% para os genótipos CC, CT e TT do polimorfismo C807T.
ConclusõesA população portuguesa encontra-se, assim, caracterizada no que respeita aos polimorfismos das principais glicoproteínas plaquetárias, o que poderá servir como ferramenta importante de estudos futuros que avaliem o papel das glicoproteínas plaquetárias na predisposição individual a condições pró-trombóticas e resposta à terapia antitrombótica.
The process of hemostasis mediated by blood platelets is complex and involves several receptor-ligand interactions. Most platelet receptors are protein complexes with two or more polypeptide subunits in the platelet membrane. Interindividual differences in platelet responsiveness are usually found, and thus it is reasonable to suggest that in certain circumstances inherited variations in platelet glycoproteins (GP) may contribute to their functional heterogeneity. In fact, three major platelet membrane adhesion receptors – the GPIb-IX-V complex, integrin α2β1 (GPIa-IIa) and integrin α2bβ3 (GPIIb-IIIa) – present genetic polymorphisms that can affect platelet responsiveness.1–6 Identification of such polymorphisms may thus be useful to assess disease predisposition and response to therapy.
The GPIb-IX-V complex mediates the initial adhesion of platelets to the subendothelial matrix under high shear stress conditions, via von Willebrand factor (vWF) binding.7 The GPIb subunit is composed of two disulfide-linked polypeptides, GPIbα and GPIbβ. GPIbα, the largest protein of the complex, contains the binding sites for vWF and α-thrombin, both platelet activator ligands.8 At least three previously described polymorphisms may influence the function and expression of the GPIbα subunit: a molecular weight polymorphism due to a variable number of tandem repeats (VNTR) within the mucin-like macroglycopeptide region of GPIbα,1 a threonine-to-methionine substitution at amino acid 145 that forms the basis of the HPA-2 platelet alloantigen system,2 and a polymorphic variation at position -5 from the ATG start codon, where either T or C is present, called the Kozak polymorphism.3
Integrin α2β1, also called the GPIa-IIa complex, is a major collagen receptor in platelets and other cell types. GPIa-IIa mediates platelet adhesion to collagen after an initial subendothelial interaction mediated by GPIb-IX-V and vWF.9 The T allele of the C807T polymorphism within the coding region of the GPIa gene (ITGA2) has been associated with high expression of the receptor and with possible platelet hyperreactivity, even though this polymorphism does not alter amino acids.4,5
The β3 integrin subunit (GPIIIa) plays a pivotal role in platelet aggregation. The β3 subunit forms a heterodimeric complex with the α2b integrin subunit (GPIIb). The major ligands for this glycoprotein are fibrinogen and vWF, when they are immobilized or in circulation after platelet activation.10 There are at least nine GPIIIa polymorphisms,11 incompatibility for the HPA-1 (PlA) alloantigens being the most common cause of fetal and neonatal alloimmune thrombocytopenia in Caucasians.6 Studies on the functional consequences of this polymorphism in thrombotic diseases have yielded conflicting results,12,13 but an increase in platelet reactivity has been reported in Pl (A2) carriers compared with Pl (A1/A1) individuals.14
In view of the importance of genetic variants in platelet function and the controversy surrounding the clinical correlation between them and prothrombotic conditions, further studies appear to be required in specific populations, that will lead to a better understanding of molecular markers with important roles in platelet function. Since there are no data regarding platelet polymorphism frequencies in the Portuguese population, the aim of this study is to assess allelic and genotypic frequencies of polymorphisms that can affect platelet function, namely the Kozak, VNTR and HPA-2 polymorphisms of the GP1BA gene (GPIbα), the PlA polymorphism of the ITGB3 gene (GPIIIa), and the C807T polymorphism of the ITGA2 gene (GPIa) in a normal Portuguese population.
MethodsPopulationThe study population consisted of 227 donors (56 male and 171 female) with ages ranging from 15 to 69 years (mean 27 years). This sample is part of a university population (students and employees) in which the majority of students are female. At the time of the sample collection donors had no clinical signs of any hemorrhagic or thrombotic disease. Clinical data regarding blood pressure, presence of diabetes, body mass index, smoking status, physical exercise and family history of cardiovascular disorders were collected but were not used to exclude any donor. Every donor was born in Portuguese territory, as were their parents. All donors gave written informed consent to the protocol, which was approved by the local ethics committee.
Genotype analysisGenomic DNA was extracted from peripheral blood cells collected by venipuncture in EDTA tubes using a PureLink™ Genomic DNA Mini Kit from Invitrogen. A region containing each polymorphism was amplified by PCR using 1 μg of DNA and 1 μM of specific primers (Table 1). Except for the VNTR polymorphism, amplicons were then digested with specific restriction enzymes (Table 1) and the digested fragments were visualized in a 2% ethidium bromide agarose gel.
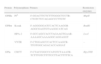
Primers and restriction enzymes used for genotypic discrimination.
| Protein | Polymorphism | Primers | Restriction enzymes |
| GPIIIa | PlA | F-GGACTTCTCTTTGGGCTCCTR-CTGTCTCCAGAGCCCTTGTC | MspII |
| GPIbα | Kozak | F-AGGGGGATCCACTCAAGGR-AGGCGAGTGTAAGGCATCAG | BsuRI |
| HPA-2 | F-GCCAGCCACCTAGAAGTGAAR-AAAAGCAAAAGGCAGGAGGT | Lwel | |
| VNTR | F-CTGGAGCCCACTCCAAGCR-TTGTGGCAGACACCAGGAT | – | |
| GPIa | C807T | F-CTACCGGCCCATGTCTAAATR-TCTTTGTCTTTTCCTTACTTTTTCA | Hpy188I |
Genotypic and allelic frequencies were calculated and Hardy-Weinberg equilibrium was tested for the five polymorphisms by means of a chi-square test using observed vs. expected genotypic frequencies for a significance level of 0.05.
ResultsTable 2 shows the allelic and genotypic frequencies obtained in the Portuguese population for the five polymorphisms. No major differences were observed compared with results from other Caucasian populations.15 Concerning GPIbα, no bb genotype for the HPA-2 polymorphism or A allele for VNTR were found. The A allele has been described mainly in Japanese and Native American populations.16 Our population is in Hardy-Weinberg equilibrium for the five polymorphisms.
Genotypic and allelic frequencies of the five studied polymorphisms.
| Protein | Polymorphism | Genotypes | Genotypic frequenciesn (%) | Alleles | Allelic frequencies |
| GPIIIa | PlA | PlA1/A1PlA1/A2PlA2/A2 | 155 (68.3)61 (26.9)11 (4.8) | PlA1PlA2 | 0.8170.183 |
| GPIbα | Kozak | TTCTCC | 180 (79.3)46 (20.3)1 (0.4) | TC | 0.8950.105 |
| HPA-2 | aaabbb | 184 (81.1)43 (18.9)0 (0.0) | ab | 0.9060.094 | |
| VNTR | BCBDCCCDDDCE | 35 (15.4)2 (0.9)160 (70.5)26 (11.5)3 (1.3)1 (0.4) | BCDE | 0.0810.8420.0750.002 | |
| GPIa | C807T | CCCTTT | 90 (39.7)114 (50.2)23 (10.1) | CT | 0.6480.352 |
There were no available data regarding the allelic frequencies of platelet glycoprotein polymorphisms in the Portuguese population. All these polymorphisms can affect platelet function. GPIIb-IIIa is the most frequent glycoprotein in the platelet surface membrane and the Leu33Pro substitution (PlA polymorphism) is located in the PSI (plexin-semaphorin-integrin) domain, which is known to bind proteins and participate in integrin activation.17 The HPA-2 polymorphism of GPIbα is near the binding site for vWF,2 VNTR affects GPIbα molecular size1 and Kozak is near the start codon, which could influence the expression rate of the gene.3 The T allele of the C807T polymorphism of GPIa has also been associated with a higher expression of this glycoprotein on the platelet surface.4
Platelet membrane glycoproteins, as key elements for normal primary hemostasis, have also been associated with platelet hyperreactivity and thrombotic mechanisms. A number of clinical studies have reported conflicting data on the association of platelet polymorphisms and increased tendency for thrombosis, and differences between populations may contribute to the contradictory results.18–20 The Portuguese population has now been characterized in terms of major platelet glycoprotein polymorphisms. Our results are of interest in future studies that aim to assess the role of platelet glycoproteins as genetic markers involved in individual predisposition to prothrombotic conditions and in patient response to therapy.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Conflicts of interestThe authors have no conflicts of interest to declare.
This project was financially supported by CESPU, CRL (AL/12/2006/CESPU, AL/11/2007/CESPU and CITS/05/2009/CESPU).