The early diagnosis of infective endocarditis (IE) is a medical challenge and a multidisciplinary approach is essential to improve its frequently fatal prognosis. Our goal was to evaluate the usefulness of [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography (18F-FDG PET) in the diagnosis of this disease.
Materials and MethodsWe prospectively assessed 43 patients (five female and 38 male) with clinical suspicion of IE between 2014 and 2017. All patients underwent transesophageal echocardiography (TEE) and an 18F-FDG PET scan, and the results were compared. A positive PET finding was defined as increased FDG uptake on cardiac valves or intracardiac devices.
ResultsOut of 43 patients with suspected IE, the diagnosis was confirmed in 30 cases (79.7%). 18F-FDG PET was positive in 24 patients, with 19 showing FDG uptake on cardiac valves (two native and 17 prosthetic) and five on cardiac devices, being concordant with echocardiographic findings in 11 cases. 18F-FDG PET sensitivity was 80%, specificity 92%, positive predictive value (PPV) 96% and negative predictive value (NPV) 66%. Echocardiography presented sensitivity, specificity, PPV and NPV of 36%, 84%, 84% and 36%, respectively.
Conclusions18F-FDG PET proved to be a sensitive technique with a high diagnostic value in patients with prosthetic valves and intracardiac devices and suspected IE. Its utility decreased dramatically in patients with suspected IE on native valves, in which TEE presented higher sensitivity and thus better diagnostic value.
O diagnóstico precoce de endocardite infecciosa (EI) é um desafio médico. Portanto, uma abordagem multidisciplinar é essencial para melhorar o prognóstico desta patologia, muitas vezes fatal. O nosso objetivo foi avaliar a utilidade da tomografia por emissão de pósitrons [18F] 2-fluoro-2-desoxi-D-glicose (18F-FDG-PET) no diagnóstico desta doença.
Materiais e métodosDe forma prospetiva, avaliámos 43 doentes (5 do sexo feminino e 38 do masculino) clinicamente suspeitos de EI entre 2014-2017. Todos os doentes foram submetidos a um ecocardiograma transesofágico (ETE) e uma PET 18F-FDG, os resultados foram posteriormente comparados. Um critério PET positivo foi definido como um aumento na captação de FDG nas válvulas cardíacas ou nos dispositivos intracardíacos.
ResultadosDos 43 doentes com suspeita de EI, o diagnóstico foi confirmado em 30 casos (79,7%). O 18F-FDG-PET foi positivo em 24 doentes, dos quais 19 demonstraram captação de FDG nas válvulas cardíacas (2 nativas e 17 protésicas) e cinco nos dispositivos cardíacos, concordando com os achados ecocardiográficos em 11 casos. A sensibilidade de 18F-FDG-PET (S) foi de 80%, especificidade (P) de 92%, valor preditivo positivo (VPP) de 96% e valor preditivo negativo (VPN) de 66%. A ecocardiografia apresentou valores de S, P, PPV e VPN de 36%, 84%, 84% e 36%, respetivamente.
ConclusõesO 18F-FDG-PET demonstrou ser uma técnica sensível com alto valor diagnóstico em doentes com suspeita de EI com próteses valvulares e dispositivos intracardíacos. A utilidade desta técnica diminui drasticamente em doentes com suspeita de EI nas válvulas nativas, nas quais a ETE apresentou maior sensibilidade e, portanto, melhor valor diagnóstico.
Infective endocarditis (IE) can be deadly if not managed early. Its poor prognosis is associated with failure to identify prosthetic and periprosthetic damage early, leading to delays in the introduction of treatments such as antibiotics and surgery.1 Therefore, this medical challenge should be managed by a multidisciplinary endocarditis team experienced in diagnosing and treating this disease.
In general, patients with suspected IE undergo various tests to reach the correct diagnosis. These may include transthoracic echocardiography, blood tests, microbiological cultures and other imaging tests such as transesophageal echocardiography (TEE) and computed tomography (CT).
The diagnosis of IE is even more difficult in patients with intracardiac devices or prosthetic valves (approximately 20% of all IE patients). In these patients, interpretation of echocardiographic findings is more difficult, and it is more difficult to apply the standard Duke criteria. This patient group also has higher mortality than those without intracardiac devices. In this context, new diagnostic tools for IE, such as cardiac nuclear magnetic resonance and [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography (18F-FDG PET), are proving their utility in the diagnosis of IE.2
18F-FDG PET has proved to be a very useful technique for detecting infection, not only due to its high sensitivity in diagnosing IE, but also by demonstrating possible septic embolisms, thereby dramatically changing the clinical approach and treatment of these patients.3,4
Our aim was to evaluate the usefulness of 18F-FDG PET in the diagnosis of this disease.
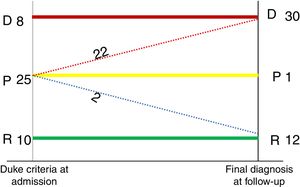
MethodsPatient eligibilityA prospective study was carried out including 43 patients with suspected IE between 2014 and 2017 in the Department of Cardiology and Nuclear Medicine of our medical center. These patients had previously been selected by a multidisciplinary endocarditis team. Twenty-five of them were previously classified as possible IE, eight as definitive IE and 10 as rejected IE, using the modified Duke criteria.3Figure 1 shows a flowchart of the progress of the 43 patients enrolled in the study period.
Of the total of 43 patients, 38 were male and 5 female. The median age was 71 years (25-88 years). The main presenting symptoms were fever and chest pain. Nineteen of the subjects had prosthetic valves (eight biological aortic valves, eight mechanical aortic valves and three mechanical mitral valves), six had native valves, seven had intracardiac devices (two with Bentall grafts and five with pacemakers), and one had both a mechanical aortic valve and a pacemaker.
Endocarditis was suspected in the presence of at least one of the following signs and symptoms with no clear origin: persistent fever >38°C; unexplained high C-reactive protein levels; positive blood cultures for the bacteria usually responsible for IE; or abnormal findings on echocardiography such as vegetation, abscess, pseudoaneurysm, intracardiac fistula, valvular perforation or aneurysm, or new partial dehiscence of a prosthetic valve.4
The final diagnosis was made according to the modified Duke criteria3 and the microbiological and anatomopathological analysis of valve tissue in patients who underwent valve replacement. All patients underwent physical examination, laboratory tests and microbiological cultures, chest X-ray, TEE and a chest CT scan 24-48 hours before 18F-FDG PET.
The indication for 18F-FDG PET was intended as an additional tool to diagnose IE, to assess valve infections and peripheral embolisms, or to rule out any other infectious focus. The mean time between TEE and 18F-FDG PET was two days (1-3 days) in hospitalized patients. In patients who had undergone previous cardiac valve surgery, no 18F-FDG PET was carried out less than three months post-intervention and none of our subjects had undergone surgery with BioGlue surgical adhesive, which is known to give false positives in 18F-FDG PET.
Imaging protocolsTo acquire a high-quality PET scan, all patients were required to follow a low-carbohydrate, fatty acid-rich diet for at least 12 hours prior to the test, to reduce physiological glucose uptake from the myocardium. Subjects also fasted for six hours before they were administered 370 MBq (10 mCi) of 18F-FDG, after checking that glucose levels were <1.8 g/l. A whole-body 18F-FDG PET scan was carried out 45 minutes after the injection and, in patients with negative or doubtful 18F-FDG uptake, a three-dimensional thoracic image was constructed. Patients with no previous medical contraindications were also intravenously administered a bolus of low-molecular weight heparin (50 IU/kg), 15 min prior to the 18F-FDG injection, to further reduce physiological uptake by increasing liver lipolysis.
All 18F-FDG PET images were fused with synchronized chest CT structural images. Both images were then reconstructed using IntelliSpace Portal 8.0 software.
Image interpretation and final diagnosisTwo physicians, specialists in nuclear medicine, assessed the corrected and uncorrected images. In the event of disagreement, a consensus was reached in discussion with a third physician. Any hypermetabolic area was considered pathological in native and prosthetic valves as well as in intracardiac devices. 18F-FDG uptake identified in the attenuation-corrected and uncorrected images was classified as focal, patchy or diffuse, and related to native, prosthetic and intracardiac devices. The intensity of 18F-FDG uptake was determined by measuring the maximum standardized uptake value (SUVmax).
The final diagnosis of IE was made by a multidisciplinary team, applying the modified Duke criteria.3 A definitive diagnosis of IE was reached in patients with two major criteria or one major plus three minor criteria or five minor criteria. A possible diagnosis of IE was made in patients with one major criterion plus one minor criterion or three minor criteria. The diagnosis was rejected when there was a firm alternative diagnosis explaining the symptoms of IE, when symptoms resolved before completion of four days of antibiotic treatment, or when patients did not meet criteria for possible IE.
Written and verbal consent was obtained from all patients, using the approved protocol at our center.
Statistical analysisStatistical analyses were performed on the data obtained using IBM SPSS version 23.0. 18F-FDG PET results fused with CT images were analyzed and compared with TEE findings using the chi-square test. The reasons for any discordant findings were also analyzed. The diagnostic performance of 18F-FDG PET compared to TEE for the diagnosis of IE in prosthetic valves, intracardiac devices and native valves (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV], and their 95% confidence intervals) was also determined and compared with the final diagnosis, reached according to the modified Duke criteria,3 considered the gold standard in this condition. Finally, anatomical (affected valves and intracardiac devices), microbiological and bacteriological findings were analyzed.
ResultsOf 43 patients with suspected IE, the final diagnosis according to the modified Duke criteria was IE in 30 cases, in whom 18F-FDG PET was positive in 24 patients, showing hypermetabolic uptake on 17 prosthetic valves, two native valves and five intracardiac devices (two Bentall grafts and three pacemakers). By contrast, TEE results were positive for IE in only 11 patients and negative in 19, being concordant with the 18F-FDG PET results in 11 cases.
The technical sensitivity of 18F-FDG PET was 80%, specificity 92%, PPV 96% and NPV 66%. TEE had sensitivity, specificity, PPV and NPV of 36%, 84%, 84% and 36%, respectively (p<0.001).
Additionally, six patients diagnosed with IE had normal cardiac 18F-FDG PET results (false negatives: 20%). Two of these patients were later diagnosed with IE secondary to pacemaker lead infection and the 18F-FDG PET was conducted only days (less than two weeks) after antibiotic therapy. Two other patients were diagnosed with IE in a native aortic valve, another with IE in both native mitral and aortic valves, and the sixth was diagnosed with an abscess in a native mitral valve.
In patients with negative or doubtful TEE results, the diagnosis of IE was reached through 18F-FDG PET (this discordance occurred in 19 out of 30 patients).
The sensitivity, specificity, PPV and NPV of these two diagnostic techniques in patients with prosthetic valves and intracardiac devices were compared to those with native valves. The diagnostic performance of 18F-FDG PET in patients with prosthetic valves and intracardiac devices was as follows: sensitivity 91%, specificity 85%, PPV 95% and NPV 75%, vs. 33%, 100%, 100% and 60% (p<0.00001) respectively, in patients with native valves. With respect to echocardiographic findings in patients with prosthetic valves and intracardiac devices, sensitivity was 25%, specificity 100%, PPV 100% and NPV 28%, compared with 83%, 66%, 71% and 80% in patients diagnosed with IE in native valves (p<0.135). The results are shown in Figure 2.
Diagnostic performance of 18F-FDG PET and transesophageal echocardiography in the diagnosis of infective endocarditis. 18F-FDG PET: [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography; NPV: negative predictive value; PPV: positive predictive value; TEE: transesophageal echocardiography.
Of the 24 cases diagnosed with IE, 16 patients had focal uptake on 18F-FDG PET, with a mean SUVmax of 2.70 g/ml, and eight patients had a patchy uptake, with a mean SUVmax of 6.67 (p=0.02, 95% confidence interval 4.71-8.49). Only one patient who did not meet all criteria for IE had positive diffuse uptake, with an SUVmax of 4.04; in this case the diagnosis was later rejected and was considered the only false positive of this study. This false result was attributed to an inflammatory response following cardiac surgery (3.5 months previously).
IE was excluded as a final diagnosis in 13 patients who did not fulfill the Duke criteria and tested negative on both echocardiography and 18F-FDG PET (true negatives), the results being concordant in 100% of cases. These patients were later diagnosed with different conditions, including bacteremia following urinary infection, systemic sepsis, septic shock, severe mitral insufficiency, pneumonia and acute coronary syndrome.
It should be added that of the 25 patients who had possible IE (24 true positives and one false positive), 22 cases were reclassified as definitive IE, two cases were rejected, and one patient remained as a possible case of IE in the follow-up. These findings are shown in Figure 3.
Following diagnosis, all patients were treated with a prolonged course of antibiotic therapy (6-12 months), carefully selected according to previous antibiogram results from valve and blood cultures.
The most common microbiological findings were Staphylococcus epidermidis, followed by Staphylococcus aureus, then Streptococcus bovis. Patients who tested negative in microbiological exams were being treated with antibiotics at the time of the tests. In these cases, the diagnosis was reached through 18F-FDG PET.
Nine deaths (30%) occurred during the study period, due to secondary complications, the most common being systemic sepsis, septic shock and heart failure.
DiscussionThe diagnosis of IE is becoming more challenging due to a variety of factors, which include the indiscriminate use of antimicrobial agents, underlying conditions in frail, elderly patients such as immunosuppression, and cardiovascular surgical procedures such as the placement of prosthetic valves and intravascular and intracardiac devices.
The results obtained in this study show that 18F-FDG PET for the diagnosis of IE in patients with prosthetic valves and intracardiac devices is a useful technique to obtain a rapid diagnosis, particularly in patients whose TEE images are doubtful, inconclusive or even negative (Figure 4).
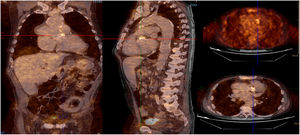
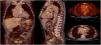
Infective endocarditis (IE) in a mechanical aortic valve in a 68-year-old male with a history of Bentall procedure and intermediate suspicion of IE. On transesophageal echocardiography, an aortic pseudoaneurysm was observed in the valvular plane with a diameter of 67 mm×44 mm and with an image of a thrombus inside. On 18F-FDG PET study, hypermetabolism was observed in the mechanical aortic valve with SUVmax of 4.49 g/ml.
We found a substantial benefit in the use of 18F-FDG PET due to its high sensitivity and specificity in the diagnosis of this disease. It should also be added that these findings have a significant impact on mortality and morbidity in these patients, due to early diagnosis and hence the ability to begin immediate treatment.5 In the European Society of Cardiology's latest guidelines for the management of IE (2015), the modified diagnostic criteria include abnormal activity around the site of prosthetic valve implantation detected by 18F-FDG PET (only if the prosthesis was implanted for >3 months) or by radiolabeled leukocyte single-photon emission computed tomography/CT.3 In summary, the sensitivity of the Duke criteria can be improved by using imaging modalities such as 18F-FDG PET/CT. Sarrazin et al.4 show that cardiac imaging plays an important role in the diagnosis and management of patients with cardiovascular implantable electronic device infection or periprosthetic valve infection. Furthermore, Saby et al. support the use of 18F-FDG PET as a major criterion for the diagnosis of IE in prosthetic valves.6
It should be noted that in our study population the usefulness of this diagnostic test decreased markedly in patients with IE in a native valve. In these patients TEE proved to be more useful. This applied to five cases in our population diagnosed with IE, without hypermetabolic uptake on 18F-FDG PET, representing false negative results. Two of these patients were diagnosed with IE due to pacemaker lead infection, one had IE in a native aortic valve and two were diagnosed with an abscess surrounding a native aortic valve.
By contrast, four of these patients had pathological TEE findings, adding a major criterion to diagnosis by the Duke criteria. These findings show that although 18F-FDG PET is a useful technique with prosthetic and cardiac devices, it has serious limitations regarding native valves and small lesions with limited visual space (<1 cm), as is the case with pacemaker lead endocarditis. Ricciardi et al.1 demonstrated the usefulness of PET/CT for IE in prosthetic valves, in contrast to its failure to detect infection in native valves, proving that this tool is not appropriate for establishing or ruling out infection of native cardiac valves.
In patients fulfilling the Duke criteria with a pathological result on TEE, the added value of 18F-FDG PET is to exclude possible septic embolisms, which can lead to complications such as permanent neurological damage or even death.2
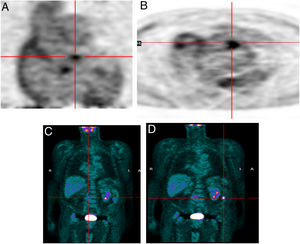
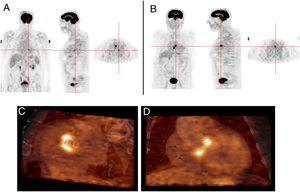
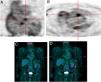
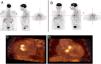
Another advantage of this technique is its ability to detect another infectious focus or even an oncological origin of the IE, revealing pre-malignant or malignant lesions, curative resection of which may be possible if they are discovered at an early stage.7 One patient in our study was referred due to neurological symptoms and was later diagnosed as having had a cerebral stroke following a septic embolism related to IE in a prosthetic aortic valve; this diagnosis was arrived at through 18F-FDG PET. Another patient who underwent 18F-FDG PET for IE had high uptake in the colon, which led to a colonoscopy being performed; adenocarcinoma of the colon was later diagnosed (Figure 5). Another patient in our study population had positive findings on TEE, a vegetation on the native mitral valve, and underwent 18F-FDG PET to exclude septic embolisms; in this patient pathological uptake was found in the native mitral and the prosthetic aortic valve (Figure 6).
Infective endocarditis in a mechanical aortic valve in a 77-year-old male with a mechanical aortic valve, referred after two weeks of fever and back pain. He presented blood cultures positive for Streptococcus bovis. Transesophageal echocardiography and prospective ECG-gated cardiac CT results were negative for endocarditis. 18F-FDG P ET study revealed hypermetabolism in the aortic valve annulus (SUVmax 2.8) (A and B) and in the lumbar column at L3-L4 level (SUVmax 6.3) (C), and radiopharmaceutical uptake is visible in the descending colon at the splenic angle (SUVmax 7.9) (D). A colonoscopy with biopsy was performed, resulting in the detection of adenocarcinoma of the colon.
Infective endocarditis in the native mitral valve and bioprosthetic aortic valve in an 88-year-old male hospitalized due to persistent fever and blood cultures positive for Streptococcus viridans. Echocardiographic results show a vegetation on the native mitral valve and no alterations in the bioprosthetic aortic valve. 18F-FDG PET images show two positive uptake foci, in (A) the native mitral valve (SUVmax 7.5) and (B–D) the prosthetic aortic valve (SUVmax 5.1).
It should be mentioned that the tendency of our results toward better outcomes regarding the diagnosis of this disease using 18F-FDG PET, thus obtaining higher sensitivity (80%), may be because the patients included in the study were selected by a specialized endocarditis team in our medical center due to a high degree of suspicion. This situation may constitute a possible selection bias in our study population, thereby inclining these findings toward perhaps excessively positive results.
With respect to SUVmax, higher values are known to be more suggestive of the presence of infection. However, there is currently no fixed cut-off that will definitively identify IE. In our study population, patients with patchy cardiac uptake had a significantly higher SUVmax than those with focal uptake, leading to statistically significant results. Nevertheless, to our knowledge, there is currently no evidence in the literature that supports these findings. Pizzi et al.8 differentiated between infection and inflammation in patients with suspected IE, using the different patterns of 18F-FDG distribution (focal, patchy and diffuse), intensity and location. It should be noted that SUVmax values are strongly affected by external factors, such as the time between radiotracer injection and PET scan, body composition and habitus, length of uptake period, plasma glucose and the partial volume effect, leading to considerable variability between patients.9
Although this technique has proved to have clear advantages, it also has some limitations. One of the most common is that it is not available in all medical centers or even countries.
Another limitation is represented by the physiological uptake of 18F-FDG from the myocardium, which can prevent accurate detection of cardiac infections. However, this problem was not found in our study population. Furthermore, cardiac uptake in 18F-FDG PET/CT results should be interpreted with caution in patients who have undergone cardiac surgery less than three months before testing, since the postoperative inflammatory response may result in non-specific 18F-FDG uptake.
In addition, many pathological conditions can mimic 18F-FDG uptake as a focal increase pattern typically observed in IE, such as the presence of active thrombi, soft atherosclerotic plaques, vasculitis, primary cardiac tumors, cardiac metastasis from a non-cardiac tumor, post-surgical inflammation, and foreign body reactions, leading to false positive results.3
18F-FDG PET also has limitations in detecting septic emboli in the brain, due to the high physiological uptake of this radiotracer in the cerebral cortex and since metastatic infections in this location are usually smaller than 5 mm, which is the spatial resolution threshold of current PET scanners.5
One of the most important limitations of this study was the unavailability of a PET/contrast-enhanced CT machine in our medical center, necessitating fusion of CT and PET images acquired at different times. Consequently, time and effort had to be expended to obtain the location of increased focus uptake in most cases. As well as establishing the added value of PET/TC, improving the diagnostic accuracy of the modified Duke criteria in 92 patients with IE and prosthetic valves and cardiac devices, Pizzi et al.2 also showed that the use of PET/contrast-enhanced CT yielded even better diagnostic performance values than PET/non-enhanced CT. However, putting aside this limitation, we can state that our results were both good and conclusive, with the advantage of lower radiation exposure for our patients and a high rate of successful diagnosis.
Another useful technique to diagnose IE, when echocardiography and 18F-FDG PET are inconclusive or even negative, is labeled-leukocyte scintigraphy, which has a high specificity for infectious diseases. However, this method is time-consuming, requires blood manipulation and its sensitivity is significantly lower than that of 18F-FDG PET, especially when patients are receiving long-term antibiotic therapy.10 Nevertheless, it can be useful as a second-line imaging diagnostic technique in patients for whom the diagnosis is unclear. This sequential strategy could prove valuable in the assessment of patients in the first three months after cardiac surgery.
In the near future, antimicrobial therapy response may be monitored using 18F-FDG PET/CT, although insufficient data are currently available to make this a general recommendation.11,12 Further research needs to be carried out to improve the diagnosis of pacemaker lead IE.
ConclusionsOur experience supports the hypothesis that 18F-FDG PET is a valuable technique in the early diagnosis of IE, particularly in patients with prosthetic valves and intracardiac devices. However, its accuracy is more limited in the assessment of IE in native valves or patients with pacemakers. TEE presents high sensitivity for diagnosing IE in native valves, but this diagnostic value decreases dramatically in patients with prosthetic valves and intracardiac devices.
Key points- •
It is well known that early diagnosis of IE is vital and that 18F-FDG PET fused with CT is extremely helpful for this purpose, especially in patients with prosthetic valves and intracardiac devices.
- •
18F-FDG PET presents serious limitations regarding native valves and small lesions such as those on pacemaker leads.
- •
Although echocardiography has high specificity, it presents serious limitations in sensitivity.
- •
An individualized approach should be adopted in order to select the diagnostic methods that are suitable for each patient's characteristics.
- •
SUVmax is helpful in diagnosing this disease, but there is currently no agreed cut-off point.
- •
18F-FDG PET may be used in the future to monitor response to antibiotic therapy.
The authors have no conflicts of interest to declare.


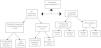


![Flowchart of the progress of the patients through the study. 18F-FDG PET: [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography; IE: infective endocarditis; TEE: transesophageal echocardiography.](https://static.elsevier.es/multimedia/08702551/0000003800000008/v2_201912121502/S0870255119305001/v2_201912121502/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)
![Diagnostic performance of 18F-FDG PET and transesophageal echocardiography in the diagnosis of infective endocarditis. 18F-FDG PET: [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography; NPV: negative predictive value; PPV: positive predictive value; TEE: transesophageal echocardiography.](https://static.elsevier.es/multimedia/08702551/0000003800000008/v2_201912121502/S0870255119305001/v2_201912121502/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)