Acute decompensated heart failure (ADHF) admissions are frequently complicated by different patterns of serum creatinine (SCr) elevation. We aimed to assess the prognostic impact of worsening renal function (WRF) based on the timing of its occurrence.
MethodsThis was a retrospective cohort of patients admitted for ADHF. Standard WRF was defined as an increase in SCr of ≥0.3 mg/dl during hospitalization. WRF timing was classified as early (within 48 hours of admission) or late (>48 hours). Acute kidney injury (AKI) at admission was defined as a rise in SCr of ≥0.3 mg/dl from outpatient baseline measurement to first measurement at admission. The primary endpoint was a composite of all-cause mortality or hospitalization for cardiovascular events at one-year follow-up.
ResultsOverall, 249 patients were included (mean age 77±11 years, 62% with preserved left ventricular ejection fraction). Early WRF occurred in 49 patients (19.7%) and was associated with a higher risk of the primary outcome (HR 2.49; 95% CI 1.66–3.73), whereas late WRF was not (p=0.411). After stratification for the presence of early WRF and/or AKI at admission, only patients with early WRF but no AKI at admission and patients with both AKI at admission and early WRF showed a higher risk of the primary outcome after multivariate Cox regression.
ConclusionEarly WRF was associated with a higher risk of the primary outcome. The timing of WRF seems to be an important factor to take into account when considering the prognostic impact of creatinine variations during hospitalization for ADHF.
Admissões por insuficiência cardíaca aguda (ADHF) são frequentemente complicadas por elevação creatinina sérica (sCr), as quais podem ter padrão variável.
ObjetivoAvaliar o impacto prognóstico do agravamento da função renal (WRF) com base no timing da sua ocorrência.
MétodosEstudo retrospetivo de coorte de doentes hospitalizados por ADHF. WRF standard foi definida como um aumento na SCr ≥0,3 mg/dL durante internamento, foi subclassificada consoante o timing da sua ocorrência em precoce (quando ocorreu nas primeiras 48 horas desde a admissão) ou tardia (quando ocorreu após as 48 h). Lesão renal aguda (AKI) à admissão foi definida como um aumento da SCr ≥0,3 mg/dL desde um valor basal ambulatório até a primeira determinação hospitalar. O endpoint primário foi um composto de mortalidade por qualquer causa ou hospitalização por eventos cardiovasculares, com um ano de follow-up.
ResultadosForam incluídos 249 doentes (média de 77±11 anos, 62% com fração de ejeção do ventrículo esquerdo preservada). WRF precoce ocorreu em 49 doentes (19,7%) e associou-se a maior risco para o outcome primário (HR 2,49; 95% CI 1,66-3,73), enquanto a WRF tardia não demonstrou essa associação (p=0,411). Após estratificação para a presença de WRF precoce e/ou AKI à admissão, apenas os doentes com WRF precoce mas sem AKI à admissão, bem como os doentes com ambas (WRF precoce e AKI à admissão), demonstraram maior risco para o outcome primário após regressão multivariável de Cox.
ConclusãoWRF precoce parece estar associada a maior risco para o outcome primário. O momento da ocorrência da WRF durante o internamento parece ser um importante fator a ter em conta quando se considera o impacto prognóstico das variações de creatinina em doentes admitidos por ADHF.
Heart failure (HF) is a major cardiovascular syndrome associated with a significant risk of mortality and hospitalization.1–3 Congestion and/or hypoperfusion can lead to organ injury, which is associated with increased mortality.4 Renal dysfunction is one of the most frequent noncardiac comorbidities in HF.5 Increased serum creatinine (SCr) levels are very common in acute decompensated HF (ADHF), with an incidence ranging from 20% to 50%.6,7
Several groups have studied the prognostic significance of worsening renal function (WRF) following initiation of diuretic therapy. However, studies have yielded conflicting results.8–11 The timing of creatinine rise may identify different subgroups of patients with different prognoses. The aim of this study was to assess the prognostic impact of the timing of WRF in patients with ADHF.
MethodsWe studied a single-center retrospective cohort of patients admitted to an HF unit due to ADHF with a ‘warm and wet’ clinical profile (type B) according to the 2016 European Society of Cardiology HF guidelines,1 between January 2014 and August 2018. Patients with chronic kidney disease (CKD) on hemodialysis, need for renal replacement therapy, need for inotropic therapy, no outpatient SCr measurement in the six months before admission, no serial SCr assessment available during hospitalization, or discharge in less than 48 hours were excluded.
During hospitalization patients underwent treatment with intravenous furosemide and started guideline-directed medical therapy as soon as indicated. Data pertaining to the index hospitalization, patient characteristics, laboratory study results and medication use, as well as events during follow-up, were extracted from electronic medical records.
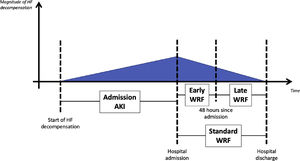
WRF was defined as an increase in SCr of ≥0.3 mg/dl based on the standard definition (from admission to any time during hospitalization).9,10 WRF timing was classified as early, when occurring within 48 hours, or late, when observed after 48 hours of hospitalization (Figure 1). Acute kidney injury (AKI) at admission was defined as a rise in SCr of ≥0.3 mg/dl from outpatient baseline measurement (up to six months before the acute episode as an outpatient) to the first measurement after patient arrival.
Patients were then stratified into four groups according to the presence of AKI at admission and the development of early WRF: group 1 (A−/W−) – no AKI at admission and no early WRF; group 2 (A+/W−) – lone AKI (AKI at admission with no early WRF); group 3 (A−/W+) – lone early WRF (no AKI at admission but with early WRF); and group 4 (A+/W+) – AKI at admission and early WRF.
The primary endpoint was a composite of all-cause mortality or hospitalization for cardiovascular events, truncated at one year after admission.
Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.12 CKD was defined as eGFR <60 ml/min/1.73 m2.
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distributions. Differences between the experimental groups were assessed by an analysis of variance (ANOVA) model, followed by the Tukey-Kramer test when findings with ANOVA were significant. Kaplan-Meier survival curves were calculated for each patient group. Univariate and multivariate analysis with Cox regression were performed to assess the prognostic value of different parameters. All reported p-values are two-tailed, with a p-value of 0.05 indicating statistical significance. The analysis was performed using IBM SPSS Statistics, version 25 (2017).
ResultsBaseline population characteristicsA total of 249 patients were included, of whom 47% were male, with a mean age of 77±11 years (Table 1). The most frequent HF etiologies were hypertensive (45.4%) and ischemic (30.5%). Overall, 155 patients (62.2%) had preserved left ventricular ejection fraction (EF) and the majority had hypertension (81.5%), atrial fibrillation (AF) (59.8%) or CKD (55.8%). At admission, patients had a median SCr and serum urea of 1.26 [0.96–1.68] mg/dl and 61 [44–96] mg/dl, respectively. Median N-terminal pro-B-type natriuretic peptide (NT-proBNP) was 4740 [2548–11400] pg/ml. Patients were hospitalized for a median of 7 [5–10] days. Over a median follow-up of 351 [73–366] days, 81 patients died (nine in-hospital, 72 during follow-up), and 125 were admitted due to a cardiovascular event. Medication at admission and at discharge for patients with reduced EF is described in Supplementary Table S1.
Baseline characteristics of the study population (n=249).
| Age, years, mean±SD | 77±11 |
| Males, n (%) | 116 (46.6) |
| LVEF, mean (±SD) | 51±17 |
| Reduced, n (%) | 66 (26.5) |
| Mid-range, n (%) | 28 (11.2) |
| Preserved, n (%) | 155 (62.2) |
| HF etiology | |
| Ischemic, n (%) | 76 (30.5) |
| Hypertensive, n (%) | 113 (45.4) |
| Valvular, n (%) | 29 (11.6) |
| Other, n (%) | 31 (12.4) |
| Comorbidities | |
| Hypertension, n (%) | 203 (81.5) |
| AF, n (%) | 149 (59.8) |
| Baseline SCr, mg/dl, median [IQR] | 1.08 [0.85–1.41] |
| Baseline eGFR (ml/min/1.73 m2), mean±SDa | 58.4±22.4 |
| Baseline eGFR <60 ml/min/1.73 m2, n (%)a | 139 (55.8) |
| Diabetes, n (%) | 92 (36.9) |
| Laboratory results at admission | |
| Hemoglobin, g/dl, median [IQR] | 11.8 [10.3–13.2] |
| Creatinine, mg/dl, median [IQR] | 1.26 [0.95–1.69] |
| Urea, mg/dl, median [IQR] | 61 [44–96] |
| NT-proBNP, pg/ml, median [IQR] | 4740 [2554–10950] |
| Sodium, mmol/l, median [IQR] | 140 [137–142] |
| Potassium, mmol/l, median [IQR] | 4.2 [3.8–4.7] |
| Medication at admission | |
| Beta-blocker, n (%) | 150 (60.2) |
| ACE inhibitor/ARB, n (%) | 166 (66.7) |
| MRA, n (%) | 58 (23.3) |
| Furosemide, n (%) | 180 (72.3) |
| Outpatient daily oral furosemide (mg), median [IQR] | 40 [0–60] |
| Oral anticoagulation, n (%) | 108 (43.4) |
| Antiplatelet therapy, n (%) | 79 (31.7) |
| Length of hospital stay, days, median [IQR] | 7 [5–10] |
ACE: angiotensin-converting enzyme; AF: atrial fibrillation; ARB: angiotensin receptor blocker; CKD: chronic renal disease; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; HF: heart failure; IQR: interquartile range; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SCr: serum creatinine; SD: standard deviation.
Overall, 90 patients (36.1%) developed WRF. These patients were significantly older and had a higher SCr at baseline and admission and longer hospital stay than those without WRF. Patients with WRF had an increased incidence of the composite endpoint in comparison to those who did not (hazard ratio [HR] 1.69 [1.15–2.48]; p=0.007) (Table 2).
Association of standard, early and late worsening renal function with the primary outcome at one year after hospitalization (univariate Cox regression).
| n (%) | HR (95% CI) | p | |
|---|---|---|---|
| Standard WRF | 90 (36.1) | 1.689 (1.152–2.477) | 0.007 |
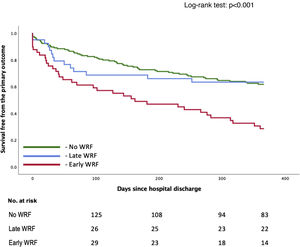
| Early WRF (≤48 h) | 49 (19.7) | 2.487 (1.659–3.728) | <0.001 |
| Late WRF (>48 h) | 41 (16.4) | 0.795 (0.453–1.395) | 0.411 |
CI: confidence interval; HR: hazard ratio; WRF: worsening renal function.
Early WRF (<48 h) was associated with a significantly higher incidence of the primary outcome (HR 2.49 [1.66–3.73]; p<0.001), whereas late WRF was not (HR 0.80 [0.45–1.40]; p=0.411), compared to patients who did not develop WRF (Table 2). Survival over follow-up is depicted in Figure 2. These subgroups are characterized in Supplementary Table S2. Patients with early WRF were older, had a longer hospital stay, and had a higher proportion of patients with preserved EF. Shorter follow-up analysis is described in Supplementary Table S3, which reveals a worse outcome for the early-WRF subgroup at one, three and six months.
Outcomes stratified by group according to admission acute kidney injury and timing of worsening renal functionDifferent patterns of creatinine variation were observed and classified into four groups.
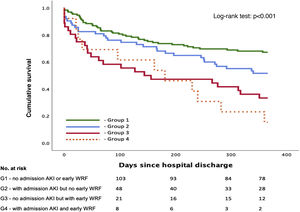
A total of 137 patients (55.0%) were included in group 1 (A−/W−), 63 (25.3%) in group 2 (A+/W−), 36 (14.5%) in group 3 (A−/W+) and 13 (5.2%) in group 4 (A+/W+). A detailed overview of baseline characteristics according to group is shown in Table 3. Group 2 (A+/W−) had significantly higher admission NT-proBNP than groups 1 (A−/W−) and 3 (A−/W+); group 3 (A−/W+) were significantly older than group 1 (A−/W−) and had a higher proportion of patients with preserved EF than groups 1 (A−/W−) and 2 (A+/W−); and group 4 (A+/W+) had higher baseline SCr, lower admission hemoglobin and longer median hospital stay than the other groups. Survival over follow-up for each of the four subgroups is depicted in Figure 3.
Baseline characteristics of each group according to the presence of acute kidney injury at admission and/or early worsening renal function (≤48 h).
| G1 (A−/W−)(n=137) | G2 (A+/W−)(n=63) | G3 (A−/W+)(n=36) | G4 (A+/W+)(n=13) | p | |
|---|---|---|---|---|---|
| Age, years, mean±SD | 75±12 | 79±10 | 82±9 | 82±6 | 0.003 |
| Males, n (%) | 62 (45.3) | 29 (46) | 19 (52.8) | 6 (46.2) | 0.882 |
| LVEF, mean±SD | 49±16 | 48±18 | 61±14 | 53±10 | 0.001 |
| Reduced, n (%) | 43 (31.4) | 19 (30.2) | 3 (8.3) | 1 (7.7) | |
| Mid-range, n (%) | 14 (10.2) | 8 (12.7) | 3 (8.3) | 3 (23.1) | |
| Preserved, n (%) | 80 (58.4) | 36 (57.1) | 30 (83.3) | 9 (69.2) | |
| HF etiology | 0.585 | ||||
| Ischemic, n (%) | 43 (31.4) | 22 (34.9) | 9 (25.0) | 2 (15.4) | |
| Hypertensive, n (%) | 59 (43.1) | 26 (41.3) | 21 (58.3) | 7 (53.8) | |
| Valvular, n (%) | 17 (12.4) | 7 (11.1) | 4 (11.1) | 1 (7.7) | |
| Other, n (%) | 18 (13.1) | 8 (12.7) | 2 (5.6) | 3 (23.1) | |
| Comorbidities | |||||
| Hypertension, n (%) | 113 (82.5) | 53 (84.1) | 28 (77.8) | 9 (69.2) | 0.575 |
| AF, n (%) | 82 (59.9) | 35 (55.6) | 23 (63.9) | 9 (69.2) | 0.755 |
| Baseline creatinine, median [IQR] | 1.04 [0.84–1.29] | 1.11 [0.87–1.49] | 1.10 [0.85–1.57] | 1.57 [1.22–1.84] | 0.003 |
| Type 2 diabetes, n (%) | 51 (37.2) | 27 (42.9) | 9 (25) | 5 (38.5) | 0.370 |
| Outpatient daily oral furosemide, median [IQR] | 40 [0–60] | 40 [0–60] | 40 [0–60] | 40 [0–70] | 0.687 |
| Laboratory results at admission, median [IQR] | |||||
| Hemoglobin, g/dl | 12.0 [10.6–13.7] | 11.9 [10.2–12.7] | 11.6 [10.4–13.1] | 9.7 [8.9–10.7] | 0.001 |
| Creatinine, mg/dl | 1.07 [0.85–1.34] | 1.65 [1.40–1.98] | 1.10 [0.86–1.32] | 2.07 [1.67–2.65] | <0.001 |
| Urea, mg/dl | 52 [40–73] | 81 [61–123] | 57 [43–79] | 110 [74–145] | <0.001 |
| NT-proBNP, pg/ml | 4380 [1974–10178] | 6952 [3418–20387] | 3500 [2316–6724] | 4380 [3171–17035] | 0.001 |
| Sodium, mmol/l | 140 [138–142] | 139 [134–142] | 141 [139–142] | 139 [132–141] | 0.090 |
| Potassium, mmol/l | 4.1 [3.6–4.5] | 4.3 [4.0–4.8] | 4.4 [3.9–4.7] | 4.3 [4.0–4.9] | 0.023 |
| Length of hospital stay, days, median [IQR] | 7 [5–8] | 7 [4–11] | 8 [7–11] | 12 [6–14] | 0.021 |
A+: AKI at admission; A−: no AKI at admission; AF: atrial fibrillation; AKI: acute kidney injury; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; IQR: interquartile range; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SD: standard deviation; W+: presence of early WRF; W−: no early WRF; WRF: worsening renal function.
After multivariate Cox regression, this stratification showed a statistically significant association with the primary outcome both for lone early WRF (A−/W+) (HR 2.34 [1.37–4.00]; p=0.002) and early WRF with AKI at admission (A+/W+) (HR 2.43 [1.18–4.00]; p=0.016) (Table 4). However, lone AKI at admission lost its statistical significance (p=0.166) after multivariate analysis.
Hazard ratios (univariate and multivariate analysis) for the primary outcome, according to selected patient characteristics.
| Composite outcome at 1 year | ||||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| No AKI at admission+no early WRF | 1.000 | 1.000 | ||
| AKI at admission+no early WRF | 1.653 (1.032–2.648) | 0.036 | 1.409 (0.868–2.287) | 0.166 |
| No AKI at admission+early WRF | 2.833 (1.711–4.692) | <0.001 | 2.339 (1.368–3.999) | 0.002 |
| AKI at admission+early WRF | 3.592 (1.843–7.001) | <0.001 | 2.429 (1.181–4.995) | 0.016 |
| Age | 1.028 (1.009–1.049) | 0.005 | 1.017 (0.995–1.040) | 0.127 |
| Gender | 1.183 (0.808–1.731) | 0.388 | ||
| LVEF | 1.011 (0.999–1.023) | 0.078 | 1.001 (0.986–1.015) | 0.912 |
| Preserved LVEF | 1.000 | |||
| Mid-range LVEF | 0.604 (0.302–1.206) | 0.153 | ||
| Reduced LVEF | 0.639 (0.397–1.028) | 0.065 | ||
| Outpatient daily furosemide dose | 1.005 (1.000–1.011) | 0.060 | ||
| Hypertension | 1.393 (0.818–2.371) | 0.222 | ||
| AF | 1.282 (0.860–1.912) | 0.222 | ||
| Baseline creatinine | 1.223 (0.562–2.662) | 0.611 | ||
| Type 2 diabetes | 1.037 (0.703–1.531) | 0.854 | ||
| Hemoglobina | 0.854 (0.775–0.941) | 0.001 | 0.909 (0.813–1.016) | 0.909 |
| NT-proBNPa | 1.000 (1.000–1.000) | 0.287 | 1.000 (1.000–1.000) | 0.655 |
| Ureaa | 1.005 (1.001–1.010) | 0.017 | ||
| Sodiuma | 0.977 (0.937–1.019) | 0.279 | ||
| Potassiuma | 1.050 (0.817–1.348) | 0.704 | ||
| Chloridea | 0.980 (0.949–1.012) | 0.222 | ||
| Ischemic etiology | 0.955 (0.628–1.451) | 0.828 | ||
| Length of hospital stay | 1.027 (0.997–1.057) | 0.073 | ||
AF: atrial fibrillation; AKI: acute kidney injury; CI: confidence interval; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
The main findings of the current analysis can be summarized as follows: (i) in patients hospitalized for ADHF, standard WRF was associated with all-cause mortality or cardiovascular events at one year; (ii) after adjustment for a set of baseline characteristics and admission laboratory results, the presence of early WRF (either alone or in association with AKI at admission) remained an independent predictor of the primary outcome.
Our findings regarding standard WRF are similar to those previously reported by other studies.8,11,13 In particular, a study by Forman et al. assessing 1004 ADHF patients, the majority of whom had heart failure with reduced ejection fraction (HFrEF), showed an association between WRF and in-hospital death.13 However, other studies showed conflicting results. A prospective study by Metra et al. that included 318 patients revealed that standard WRF was not associated with death or HF hospitalization,9 although a sizable proportion of these patients were treated with dopamine (22%) or inotropes (9%), which may have influenced SCr variation in response to diuretic therapy and renal hemodynamics.9 Similarly, Cowie et al. studied 299 patients with acute decompensated HFrEF and showed no statistically significant association between standard WRF and mortality at six months.10 The majority of these patients developed late WRF (median time to WRF was four days), which, in our study, was not associated with worse outcomes.10 Other studies have been published on WRF, using a wide variety of definitions, hindering the comparability of the results.11,14–16
Another important finding of our work is that early WRF was associated with worse outcomes not only at one year of follow-up, but also at shorter follow-ups, in comparison to late WRF. This underscores the importance of this adverse event in patient hospitalization, which may be explained by the presence of more severe disease, associated with lower cardiac and/or renal reserve, limiting the compensatory response that is usually triggered.
To the best of our knowledge, this is the first study to assess the prognostic impact of WRF timing in patients with ADHF. We aimed to analyze whether early deterioration of renal function, specifically within 48 hours of admission, was a marker of worse prognosis. We employed a 48-hour cut-off for WRF, as this excluded several confounding factors associated with SCr variations during hospitalization. For example, excessive diuretic therapy or initiation/uptitration of renin-angiotensin-aldosterone inhibitors frequently occur during hospitalization and may raise SCr. This type of WRF (often labeled pseudo-WRF) does not seem to be associated with worse outcomes.17 This idea is further corroborated by our study, since we found no association between late WRF (>48 hours after admission) and worse outcomes. Hence, the timing of SCr elevation seems to have an impact in differentiating risk and its pathophysiology.
Furthermore, stratification of patients according to AKI at admission and early WRF status shows that lone early WRF (A−/W+) was associated with a higher risk for the primary outcome. Although this was in a population that was older, had longer hospital stay and had a higher proportion of preserved EF, the prognostic impact remained after controlling for various relevant factors. Conversely, lone AKI at admission (A+/W−) was not an independent predictor of the primary outcome after multivariate Cox regression. These results seem to differ from the work of Shirakabe et al., who concluded that, in a cohort of 1083 ADHF patients, lone WRF (in the first five days) was not associated with higher risk of all-cause death.8 However, as well as using a broader WRF definition, this cohort of patients had more severe disease, and were admitted to an intensive care unit with frequent need for intravenous inotropes/vasopressors, and had a considerably longer hospital stay.8
Each of these subtypes of WRF seems to identify a different set of patients. Changes in renal function in patients with HF are complex and multifactorial. Multiple mechanisms can play a part in WRF, including renal hypoperfusion, activation of the renin-angiotensin-aldosterone and sympathetic systems, and venous congestion.18,19 The clinical importance of each mechanism is likely to vary from patient to patient.8 AKI at admission may also be related to progression of renal disease in the months preceding hospitalization as well as to the cumulative congestion these patients usually develop.4 On the other hand, early WRF may be related to reduced cardiac output and low cardiac and/or low renal reserve, which result in elevation of SCr after initiation of diuretic therapy.4 Consequently, the prognosis varies, depending mostly on the main underlying mechanism of renal dysfunction rather than SCr, which is a limited surrogate biomarker of renal function.
In our opinion, acute renal dysfunction should be differentiated according to various factors. As well as considering absolute variation in SCr, the timing of its appearance should be taken into account. We speculate that early WRF after initiation of diuretic therapy may be a marker of more severe underlying cardiac and/or renal disease, thus identifying patients in whom the usual compensatory mechanisms cannot counteract the hemodynamic effects of diuretics, unveiling reduced organ reserve.
LimitationsThe present study has some limitations. Most patients were not admitted directly to the HF clinic. There was some variation between patients in the time from arrival at the emergency department until admission to the HF clinic. Nonetheless, the vast majority were admitted to our unit within 24 hours. Also, information regarding diuretic doses administered was not available.
Mechanisms associated with creatinine variation during hospital stay were not directly measured, since the data were collected retrospectively. Studying these factors prospectively would shed more light on their mechanisms and prognostic impact.
This was a single-center retrospective study. Thus, it should be viewed as hypothesis-generating, due to the possible presence of unmeasured confounding factors and selection bias.
The low number of patients influences the power of this study. Consequently, associations with smaller effect sizes may not be apparent in this analysis.
Nonetheless, our study supports the findings of previous investigations, reinforcing the importance of timing patterns of WRF as a prognostic predictor.
ConclusionIn this study, the presence of WRF, particularly when occurring within 48 hours of initiation of diuretic therapy, was associated with a higher risk of death from any cause or hospitalization for cardiovascular events. The timing of WRF appears to be an important characteristic to take into account when considering the prognostic impact of creatinine variation during hospitalization for ADHF.
Authors’ contributionsConception and design: JP, GC, BR, IA, CF; data collection: JP, GC, LL, ST, MR, MIS, MM, SM; data analysis: JP, GC, BR; drafting: JP, GC, BR; revising: JP, GC, LL, ST, MR, MIS, MM, SM, BR, CR, IA, CF. All authors read and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.