Acromegaly is a relatively rare chronic hormonal disease resulting in disfigurement. In 90% of cases, acromegaly is caused by a benign pituitary monoclonal human growth hormone-secreting tumor. The aim of the present study was to determine the presence of left ventricular (LV) deformation abnormalities using three-dimensional speckle-tracking echocardiography in a group of acromegalic patients.
MethodsThirty-eight acromegalic patients were involved in the study. Thirteen patients were excluded due to inadequate image quality. The mean age of the remaining patients was 57.2±13.6 years and seven were male. Their data were compared to an age- and gender-matched control population, which consisted of 34 healthy volunteers (mean age: 52.7±4.9 years, 15 male).
ResultsGlobal and mean segmental LV radial strain (RS) (33.2±13.4% vs. 25.2±10.8%, p=0.01 and 36.0±12.1% vs. 28.2±10.0%, p=0.009, respectively) proved to be significantly higher in acromegaly compared to controls. Active acromegalic patients had significantly higher global and mean segmental LV-RS (35.5±14.4% vs. 25.2±10.8%, p=0.03 and 37.9±13.3% vs. 28.2±10.0%, p=0.03, respectively) compared to controls. Between the active and inactive acromegaly groups, only basal LV circumferential strain (-30.2±4.8% vs. -26.7±4.1%, p=0.02) was found to be significantly different.
ConclusionThe presented clinical, demographic, therapeutic and echocardiographic features demonstrate that active acromegaly is associated with enhanced LV RS as compared to healthy controls and those with inactive acromegaly.
A acromegalia é uma doença hormonal crónica relativamente rara, associada a dismorfismos somáticos. Em 90% dos casos, ela é causada por um tumor benigno monoclonal da hipófise, secretor de hormona do crescimento. O objetivo do presente estudo foi detetar a presença de alterações da deformação miocárdica do ventrículo esquerdo (VE) através de ecocardiografia tridimensional por speckle tracking num grupo de doentes portadores de acromegalia.
MétodosForam incluídos 38 doentes portadores de acromegalia. Treze foram excluídos devido à má qualidade de imagem. A idade média dos restantes foi de 57,2±13,6 anos (7 homens). Os seus dados foram comparados com uma população controlo emparelhada por idade e género, que compreendeu 34 voluntários saudáveis (idade média: 52,7±4,9 anos, sendo 15 indivíduos do sexo masculino).
ResultadosO strain radial (SR) global e segmentar VE (respetivamente 33,2±13,4% versus 25,2±10,8%, p=0,01 e 36,0±12,1% versus 28,2±10,0%, p=0,009) foi significativamente superior nos casos de acromegalia em relação aos casos controlo. Os doentes portadores de acromegalia ativa tiveram um SR global e segmentar significativamente superior (35,5±14,4% versus 25,2±10,8%, p=0,03 e 37,9±13,3% versus 28,2±10,0%, p=0,03, respetivamente), quando comparados com os grupos controlo. Entre os grupos portadores de acromegalia ativos e inativos, só foi considerado significativamente diferente o strain circunferencial basal VE (-30,2±4,8% versus -26,7±4,1%, p=0,02).
ConclusõesCom base nas características clínicas, demográficas, terapêuticas e ecocardiográficas apresentadas, a acromegalia ativa apresenta SR-VE aumentado em relação à população saudável e ao grupo de portadores de acromegalia inativa.
Acromegaly is a relatively rare chronic hormonal disease resulting in disfigurement. In 90% of cases, acromegaly is caused by a benign pituitary monoclonal human growth hormone (HGH)-secreting tumor.1 Elevation of serum HGH causes an elevation in insulin-like growth factor 1 (IGF-1), and these hormonal changes cause a wide range of clinical symptoms and comorbidities including metabolic, respiratory, other endocrine and cardiovascular diseases.2,3 Of these cardiovascular comorbidities, hypertension and left ventricular (LV) hypertrophy are the most common, but serious valvular regurgitation and heart failure can also develop. A higher incidence of cardiac arrhythmias, most commonly atrial fibrillation, has frequently been reported in acromegaly.4,5
Three-dimensional (3D) speckle-tracking echocardiography (STE) (3D-STE) is a new, non-invasive, robust and validated tool offering clinicians a new imaging modality. 3D-STE is capable of measuring various global, segmental and regional strain values through LV modeling.6,7 To the best of the authors’ knowledge, there have been no 3D-STE studies on LV deformation abnormalities in acromegaly. Therefore, the aim of the present study was to determine the presence of LV deformational abnormalities using 3D-STE in a group of acromegalic patients.
MethodsPatient populationThirty-eight acromegalic patients were included in the present study. Thirteen were excluded due to inadequate image quality. The mean age of the remaining patients was 57.2±13.6 years and seven were male. Their data were compared to an age- and gender-matched control population, which consisted of 34 healthy volunteers (mean age 52.7±4.9 years, 15 male). The diagnosis of acromegaly was based on current clinical standards: typical clinical features, elevated HGH levels, and elevated IGF-1 levels insuppressible with an oral glucose tolerance test.5 The acromegalic group was further divided based on the activity of the disease: if serum HGH and/or IGF-1 concentration was over the diagnostic threshold, acromegaly was considered to be active.5,8 Our study was approved by the human research ethics committee of our institution and complied with the Declaration of Helsinki. All of the patients and healthy volunteers gave informed consent. Our acromegalic datasets came from the MAGYAR-Path Study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases), which was developed to assess the diagnostic and prognostic value of 3D-STE-derived parameters (‘Magyar’ means ‘Hungarian’ in the Hungarian language).
Two-dimensional Doppler and tissue Doppler echocardiographyAll healthy volunteers and acromegalic patients underwent a complete two-dimensional (2D) transthoracic echocardiographic study using a Toshiba ArtidaTM imaging system (Toshiba Medical Systems, Tokyo, Japan) with a PST-30SBP phased-array transducer (1-5 MHz). The examinations were performed according to current clinical standards. In all cases LV dimensions, volumes and ejection fraction (LVEF) and left atrial (LA) dimensions were measured and complete 2D Doppler and tissue Doppler studies were performed.9
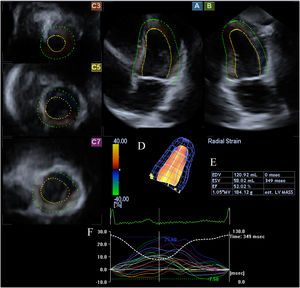
Three-dimensional speckle-tracking echocardiographyFor the 3D-STE examinations, the same Toshiba Artida™ system was used, with a PST-25SX matrix- array transducer (Toshiba Medical Systems, Tokyo, Japan).7 In each case, a pyramid-shaped full-volume 3D dataset was obtained. The complete dataset consisted of six wedge-shaped subvolumes, which were recorded during six RR intervals and a single breath-hold. The subvolumes were set to be as narrow as possible to improve spatial resolution, thus improving later endocardial border delineation. The recorded datasets were analyzed offline using the supplied 3D Wall Motion Tracking software, version 2.7 (Toshiba Medical Systems, Tokyo, Japan). From the datasets, the software automatically reconstructed apical 4-chamber and 2-chamber views, then the reader specified the three cross-sectional planes manually, using the guide planes to help standardize LV measurements. After setting up the planes, the reader then specified the septal and lateral parts of the mitral annulus and the LV apex, after which the software automatically detected the endocardial border and created a 3D LV cast (Figure 1).
Three-dimensional speckle-tracking echocardiographic study of an acromegalic patient. Apical 4-chamber view (A) and apical 2-chamber view (B) are automatically selected by the software. Cross-sectional planes are at the apical (C3), midventricular (C5) and basal (C7) left ventricular (LV) levels. The three-dimensional LV model (D) and the corresponding volumetric parameters (E) are shown along with segmental LV radial strain curves (F).
3D-STE was used to measure LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LVEF and LV mass based on the above-mentioned LV cast.
Three-dimensional speckle-tracking echocardiography-derived left ventricular strain assessments3D-STE strain measurements were performed on the LV cast using the 16-segment LV model.10 In each segment, three unidirectional strain parameters were assessed: radial (RS) (thickening and thinning of the segment), longitudinal (LS) (lengthening and shortening of the segment) and circumferential (CS) (widening and narrowing of the segment). Based on these strain parameters, the software calculated the following complex strains: area (AS) (combination of LS and CS) and 3D (3DS) (combination of RS, LS and CS).
Statistical analysisAll data are reported as mean ± standard deviation. Significance was established for p values less than 0.05. The Shapiro-Wilks test was used to check for normal distribution. Levene's test for equality of variances was used to test homogeneity of variance. For normally distributed datasets, the two-tailed Student's t test was used for comparisons, however if the dataset did not follow a normal distribution, the Mann-Whitney-Wilcoxon test was used. For categorical variables, Fisher's exact test was performed. To establish significant relationships between independent variables, Pearson's correlation coefficients were calculated. The statistical analysis was performed using RStudio (RStudio Team, RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, 2015). For offline data extraction and analysis, a commercial software package was used (MATLAB 8.6, The MathWorks Inc., Natick, MA, 2015).
ResultsClinical and demographic characteristics of patients with acromegalySignificant differences were identified between acromegalic patients and healthy controls regarding the prevalence of hypertension (p<0.0001), hypercholesterolemia (p<0.0001) and diabetes (p=0.01) (Table 1).
Demographic, clinical and two-dimensional echocardiographic characteristics of patients with acromegaly and of controls.
| Controls (n=34) | Acromegalic patients (n=25) | p | |
|---|---|---|---|
| Risk factors | |||
| Age, years | 52.7±4.9 | 57.2±13.6 | 0.07 |
| Male gender, % | 15 (44) | 7 (28) | 0.27 |
| BSA, m2 | 1.87±0.23 | 2.05±0.29 | 0.01 |
| Hypertension, % | 0 (0) | 15 (60) | <0.0001 |
| Hypercholesterolemia, % | 0 (0) | 12 (48) | <0.0001 |
| Diabetes, % | 0 (0) | 5 (20) | 0.01 |
| Hypophysectomy, % | 0 (0) | 9 (36) | 0.0002 |
| 2D echocardiography | |||
| LA diameter, mm | 38.8±4.4 | 42.2±6.1 | 0.02 |
| LA diameter/BSA, mm/m2 | 20.7±2.6 | 20.6±3.2 | 0.9 |
| LA volume, ml | 48.8±16.5 | 54.1±18.0 | 0.4 |
| LA volume/BSA, ml/m2 | 24.1±8.1 | 28.5±9.5 | 0.8 |
| LVEDD, mm | 47.7±3.3 | 51.3±5.3 | 0.006 |
| LVEDD/BSA, mm/m2 | 25.8±3.1 | 25.6±4.2 | 0.9 |
| LVEDV, ml | 107.0±20.3 | 129.9±28.7 | 0.002 |
| LVEDV/BSA, ml/m2 | 58.6±9.8 | 64.9±16.5 | 0.1 |
| LVESD, mm | 31.6±3.0 | 31.6±4.7 | 0.95 |
| LVESD/BSA, mm/m2 | 17.0±2.6 | 15.9±2.6 | 0.2 |
| LVESV, ml | 37.7±9.0 | 41.9±14.7 | 0.2 |
| LVESV/BSA, ml/m2 | 19.1±6.3 | 21.3±6.8 | 0.3 |
| IVS, mm | 9.4±1.3 | 10.3±1.6 | 0.02 |
| LV posterior wall, mm | 9.6±1.6 | 10.8±1.8 | 0.007 |
| E, cm/s | 71.5±18.9 | 65.3±14.0 | 0.3 |
| A, cm/s | 70.3±18.0 | 79.3±16.1 | 0.05 |
| E/A | 1.06±0.31 | 0.85±0.21 | 0.004 |
| LVEF, % | 64.5±3.6 | 67.6±7.2 | 0.1 |
2D: two-dimensional; A: late transmitral flow velocity; BSA: body surface area; E: early transmitral flow velocity; IVS: interventricular septal thickness; LA: left atrial; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume.
The active acromegaly subgroup consisted of 14 patients (mean age: 58.6±14.6 years, five males). Their mean serum HGH level was 5.5±3.9 ng/ml, IGF-1 level was 502.3±366.0 ng/ml and IGF-1 index (IGF-1 level/upper limit of normal) was 2.0±1.1. The inactive acromegaly group included 11 patients (mean age: 54.0±12.9, 2 males). Their mean serum HGH level was 4.5±8.1 ng/ml, IGF-1 level was 216.9±129.0 ng/ml and IGF-1 index was 0.9±0.6.
Two-dimensional echocardiographic dataSignificant differences were seen in LA diameter (p=0.02), LV end-diastolic diameter (LVEDD) (p=0.006) and volume (LVEDV) (p=0.002), interventricular septal thickness (p=0.02), and LV posterior wall thickness (p=0.007) between the acromegalic group and healthy controls. Body surface area (BSA) was also significantly different between controls and acromegalic patients (p=0.01). Furthermore, late transmitral flow velocity A(p=0.05) and E/A ratio (p=0.004) also differed significantly between the two groups (Table 1).
Three-dimensional speckle-tracking echocardiography-derived left ventricular volumetric parameters3D-STE-derived LVEDV (p=0.0003) and LVESV (p=0.005) proved to be significantly different between all the acromegalic patients and controls. LVEDV (p=0.006) and LVEF (p=0.02) differed significantly between the active acromegaly subgroup and controls, while LVEDV (p=0.01) and LVESV (p=0.01) were significantly different between the inactive disease subgroup and controls. LVEF (p=0.02) was significantly higher in active than in inactive disease (Tables 2 and 3).
Comparison of three-dimensional speckle-tracking echocardiography-derived global and mean segmental left ventricular peak strain parameters between acromegalic patients and controls.
| Controls (n=34) | Acromegalic patients (n=25) | Active disease (n=14) | Inactive disease (n=11) | |
|---|---|---|---|---|
| 3D-STE-derived volumetric parameters | ||||
| LVEDV, ml | 80.5±20.2 | 107.6±28.9a | 108.5±34.6a | 101.1±23.3a |
| LVESV, ml | 34.9±10.6 | 45.4±14.9a | 43.7±17.7 | 45.3±12.7a |
| LVEF, % | 56.7±7.1 | 58.1±6.1 | 60.2±5.8a,b | 55.6±5.8 |
| Global strains | ||||
| Radial, % | 25.2±10.8 | 33.2±13.4a | 35.5±14.4a | 29.7±11.3 |
| Circumferential, % | -26.7±6.1 | -28.1±4.5 | -29.3±4.1 | -27.0±4.9 |
| Longitudinal, % | -16.0±2.7 | -15.8±3.4 | -15.5±3.1 | -16.1±3.9 |
| 3D, % | 27.6±10.7 | 31.3±11.8 | 32.5±13.8 | 29.1±8.3 |
| Area, % | -39.3±5.8 | -39.9±5.2 | -40.8±4.7 | -39.3±6.0 |
| Mean segmental strains | ||||
| Radial, % | 28.2±10.0 | 36.0±12.1a | 37.9±13.3a | 33.1±9.9 |
| CS, % | -28.1±5.9 | -29.1±4.3 | -30.3±4.1 | -28.2±4.4 |
| LS, % | -16.9±2.6 | -17.0±3.4 | -16.7±3.0 | -17.5±4.0 |
| 3DS, % | 30.2±9.8 | 33.7±10.8 | 34.6±12.5 | 31.6±7.6 |
| AS, % | -40.4±5.6 | -41.2±5.3 | -42.1±4.7 | -40.6±6.1 |
p<0.05 vs. inactive disease.
3D: three-dimensional; 3D-STE: three-dimensional speckle-tracking echocardiography; 3DS: three-dimensional strain; AS: area strain; CS: circumferential strain; LS: longitudinal strain; LVEDV: end-diastolic volume, LVESV: end-systolic volume, LVEF: left ventricular ejection fraction; RS: radial strain.
Comparison of three-dimensional speckle-tracking echocardiography-derived regional left ventricular peak strain parameters between acromegalic patients and controls.
| Controls (n=34) | Acromegalic patients (n=25) | Active disease (n=14) | Inactive disease (n=11) | |
|---|---|---|---|---|
| RS basal, % | 34.3±14.8 | 44.6±19.2a | 46.9±20.7a | 40.2±16.8 |
| RS mid, % | 29.6±12.1 | 34.6±12.8 | 35.8±14.2 | 32.1±10.1 |
| RS apex, % | 16.9±8.3 | 25.6±10.1a | 27.9±10.6a | 23.9±10.4 |
| CS basal, % | -26.1±5.8 | -28.9±4.9 | -30.2±4.8a,b | -26.7±4.1 |
| CS mid, % | -28.4±7.4 | -28.5±5.0 | -29.3±5.1 | -27.8±5.2 |
| CS apex, % | -30.6±12.6 | -30.6±10.0 | -31.7±9.2 | -31.1±9.7 |
| LS basal, % | -20.8±5.0 | -19.1±5.5 | -18.3±6.1 | -19.3±4.4 |
| LS mid %) | -13.6±4.1 | -14.7±3.6 | -15.0±2.9 | -15.0±4.7 |
| LS apex, % | -16.0±5.5 | -17.3±7.7 | -16.9±8.7 | -18.4±6.4 |
| 3DS basal, % | 37.1±13.9 | 44.3±17.4 | 45.9±19.5 | 40.7±13.9 |
| 3DS mid, % | 30.7±11.9 | 30.3±12.5 | 31.2±14.2 | 28.1±9.1 |
| 3DS apex, % | 19.2±9.3 | 21.8±10.8 | 23.0±10.9 | 21.5±11.4 |
| AS basal, % | -40.6±6.8 | -41.6±7.1 | -42.0±7.8 | -40.0±5.5 |
| AS mid, % | -38.8±7.5 | -39.0±6.2 | -40.4±5.4 | -38.3±7.5 |
| AS apex, % | -42.7±14.4 | -43.8±12.8 | -44.6±12.2 | -45.0±12.5 |
Global and mean segmental RS (p=0.01 and p=0.009, respectively) were significantly higher in acromegalic patients compared to controls. Among regional strain parameters, basal and apical RS (p=0.04 and p=0.0004, respectively) were significantly different between acromegalic patients and controls. Patients with active disease had significantly higher global and mean segmental RS (p=0.03 and p=0.03, respectively) compared to controls; in addition, basal and apical RS (p=0.04 and p=0.002, respectively) and basal CS (p=0.01) were significantly higher compared to controls. In patients with inactive disease, no significant differences were detected in LV strain parameters compared to the control group. Between active and inactive disease groups, only basal CS (p=0.02) was found to be significantly different (Tables 2 and 3).
Correlation between hormone levels and three-dimensional speckle-tracking echocardiography-derived left ventricular volumetric and strain parametersIn the overall acromegaly group, there was a significant negative correlation between HGH levels and apical 3DS (r=-0.40, p=0.05). No significant correlation was detected between any hormone level and LV strains in the active disease group. In the inactive disease group, IGF-1 index correlated significantly with midventricular RS (r=0.60, p=0.05), global and mean segmental RS (r=0.66, p=0.03 and r=0.66, p=0.03, respectively) and global 3DS (r=0.61, p=0.05). Furthermore, there was a significant correlation between mean segmental RS and IGF-1 levels (r=0.61, p=0.05) in this group.
DiscussionAcromegaly is a severe chronic hormonal disease that can affect almost any organ system through elevated HGH and IGF-1 levels. These effects are especially marked in the cardiovascular system, resulting in numerous cardiovascular comorbidities, the most common of which is hypertension. Although the pathophysiology is not fully understood, it is hypothesised that circulatory volume increases due to sodium retention and consequent plasma expansion.11–13 Furthermore, acromegaly is associated with cardiomyopathy, resulting in severe heart failure in terminal stages. In the early stages of acromegaly, LV hypertrophy develops due to exposure to elevated serum HGH levels. As acromegalic cardiomyopathy develops, Ca2+ hypersensitivity enhances myocardial contractility, resulting in a hyperkinetic syndrome. As it progresses, LV hypertrophy may become sufficiently severe to impair LV diastolic filling. In the terminal stages, both systolic and diastolic function may decrease due to extracellular collagen deposition.14–17 In addition, valvular regurgitation and arrhythmias are more common among acromegalic patients.18,19
In the present study, 3D-STE was used to assess global and regional LV strain parameters in acromegalic patients. Strain imaging using 3D-STE has been validated in comparisons with two-dimensional speckle-tracking echocardiography (2D-STE), cardiac magnetic resonance imaging and tissue Doppler imaging.6,10,20–22 It has been reported that 3D-STE-derived LV strain values are somewhat lower than those from 2D-STE.6 Notable advantages of this method compared to other modalities are its non-invasive nature, angle independence, possibility of 3D imaging and lower cost.10
To the best of the authors’ knowledge, there have been no 3D-STE studies on LV strain parameters in the acromegalic population. There has been only one study which used 2D-STE to assess global LV longitudinal strain in patients with active acromegaly, by Volschan et al., who reported no significant differences in global LV-LS compared to healthy controls.23 Our group has previously reported increased aortic stiffness in acromegaly as well as reduced LV rotation and twist in both active and inactive acromegaly. It could be hypothesized that these changes are the consequences of extracellular collagen deposition.24,25
Results of the 2D echocardiography study in our population showed that in patients with acromegaly, the LV was dilated, LV walls were significantly thicker and there was impaired LV diastolic function without LV systolic dysfunction. Of the 3D-STE-derived LV volumetric parameters, LVEDV was significantly increased regardless of disease activity. In patients with active acromegaly, LVEF was higher compared to the control group, and was significantly higher than in the inactive acromegaly group. Global and mean segmental LV-RS were significantly higher in cases of active disease compared to both the healthy controls and those with inactive disease. It should be noted that LV strain values were normal in patients with inactive acromegaly. Based on these findings, it could be hypothesized that enhanced regional strain may be a compensatory mechanism offsetting the impaired LV rotation and twist demonstrated recently.25 Our results suggest that LV deformation differences are reversible with proper treatment, but not LV dilation. Further investigations are warranted to assess why LV rotation and twist are impaired but LV wall contractility is not. In addition, LV-RS appears to compensate for the loss of LV twist.
LimitationsThere are several limitations affecting the present study. Since acromegaly is a rare disease, the study population is relatively small. The duration of exposure to high serum HGH and IGF-1 concentrations was not taken into account in the analysis, and the possible effects of drugs used in the treatment of acromegaly were not examined. 3D-STE as a novel modality suffers from several limiting technical factors: it has lower spatial and temporal resolution than 2D-STE, and the full-volume dataset requires six identical RR intervals, which can result in so-called stitching noise.26 It has also been reported that LV strain parameters show some vendor dependency.27 Finally, the greater proportion of women in the control group could partially explain differences in heart dimensions between the groups.
ConclusionThe presented clinical, demographic, therapeutic and echocardiographic features demonstrate that active acromegaly is associated with enhanced LV-RS as compared to healthy controls and those with inactive acromegaly.
Ethical standardsThe authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the 1975 Helsinki Declaration, revised in 2008, and have been approved by the institutional review board of the University of Szeged.
Author contributionsÁrpád Kormányos – study design, writing manuscript, patient management, performing 3D-STE examinations.
Péter Domsik – patient management, performing 3D-STE examinations.
Anita Kalapos – patient management, performing 3D-STE examinations.
Nándor Gyenes – patient management, performing 3D-STE examinations.
Zsuzsanna Valkusz – supervision.
Csaba Lengyel – supervision.
Tamás Forster – supervision.
Attila Nemes – study design, writing manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors.