Ablation of multifocal premature ventricular complexes (PVCs) is challenging. Activation mapping can be performed for the predominant morphology, but may be useless for other less prevalent ones. We aimed to describe the efficacy of an automated pace-mapping software-based ablation strategy for ablating the site of origin of multiple PVC locations.
MethodsConsecutive patients referred for ablation of multifocal PVCs were prospectively enrolled. Spontaneous PVC templates were recorded and a detailed pace-mapping map was generated to spot the site of origin of PVCs.
ResultsA total of 47 PVCs were targeted in 21 patients (five and 16 patients with three or two PVCs morphologies each, respectively). Detailed pace-mapping comprising 73.5±41.6 different pacing locations was performed (best matching 97.2% [IQR 95.9-98.3%] similar to the clinical PVC). Activation points were acquired if possible, although ablation was only based on pace-mapping in 13 (27.6%) foci. Complete acute procedural success was obtained in 14 (66.7%) patients, while one PVC morphology was deliberately not ablated in five patients (23.8%). After 12.3±9.4 months of follow-up, PVC burden decreased from 24.4±10.4% to 5.6±5.0% (p<0.001). Interestingly, patients with acute procedural failures or with some PVCs deliberately not targeted during the procedure also experienced a significant decrease in PVC burden (30.0±8.9% to 11.9±3.5%, p=0.002).
ConclusionQuantitative morphology-matching software can be used to obtain a detailed map identifying the site of origin of each single PVC, and successful ablation can be performed at these sites, even if activation points cannot be obtained due to the paucity of ectopic beats.
A ablação de extrassístoles ventriculares (ESV) multifocais constitui um desafio. O mapeamento de ativação pode ser realizado para a morfologia predominante, podendo, no entanto, ser inútil nas morfologias menos predominantes. Pretendemos descrever a eficácia de uma estratégia de ablação baseada num software automático de pace-mapping para ablação do local de origem de múltiplas localizações de ESV.
MétodosDoentes consecutivos indicados para ablação das ESV multifocais foram incluídos prospetivamente. Foram gravados templates espontâneos de ESV e foi gerado um mapa detalhado de pace-mapping para detetar o local de origem das ESV.
ResultadosQuarenta e sete ESV foram estudadas em 21 doentes (5 e 16 doentes com três morfologias e com duas morfologias de ESV, respetivamente). Foi concebido um mapa detalhado de pace-mapping compreendendo 73,5 ± 41,6 locais diferentes de pacing [melhor combinação 97,2% (IQR 95,9%-98,3%) semelhante à ESV clínica). Foram captados pontos de ativação, embora a ablação se tenha baseado apenas no pace-mapping em 13 focos (27,6%). Verificou-se um sucesso agudo completo do procedimento em 14 doentes (66,7%), embora uma morfologia de ESV não tenha sido deliberadamente intervencionada em 5 doentes (23,8%). Após 12,3±9,4 meses de seguimento, a carga de ESV diminuiu de 24,4±10,4% para 5,6±5,0% (p<0,001). Os doentes com insucesso agudo do procedimento ou com algumas ESV deliberadamente não intervencionadas durante o mesmo também sofreram uma diminuição significativa da carga de ESV (30,0±8,9% a 11,9±3,5%, p=0,002).
ConclusãoUm software quantitativo adequado à morfologia de ESV para obter um mapa detalhado com o seu local de origem pode ser utilizado, sendo a ablação bem-sucedida nestes locais, mesmo que não seja possível obter pontos de ativação devido à escassez de batimentos ectópicos.
Ablation of premature ventricular contractions (PVCs) has become a widely used treatment for patients with symptomatic PVCs or suspected of having left ventricular ejection fraction (LVEF) impairment due to PVCs, so-called PVC-induced cardiomyopathy.1 The success rate of ablation depends on many factors, including the ability to identify the site of origin2,3 and the burden of PVCs at the onset of the procedure.4 The number of PVC foci to ablate may also impact procedural success. In most cases, a single PVC needs to be targeted, but in some instances, multifocal PVCs need to be ablated, either arising from a single focus with different myocardial exit sites, or arising from multiple different ventricular sites.
The strategy for PVC ablation can be based on mapping the earliest activation or on dedicated automated pace-mapping software such as PaSo® (CARTO 3, Biosense Webster Inc., Diamond Bar, CA, USA). For patients with multifocal PVCs, activation mapping can be performed for the predominant morphology, but may be useless for other less prevalent ones. Morphology-matching software may then be the only solution for determining the site of origin of multiple PVC locations.
Thus, we aimed to describe the workflow and to determine the efficacy of an automated pace-mapping-based ablation strategy for multifocal PVCs.
MethodsStudy populationConsecutive patients referred to our tertiary center for ablation of multifocal PVCs between June 1, 2016 and December 31, 2018 were prospectively enrolled. Patients were included if they had symptomatic PVCs or asymptomatic PVCs associated with impaired LVEF (<50%), suggesting PVC-induced cardiomyopathy. Patients were excluded if they had had prior ablation of ventricular arrhythmia (either PVC or ventricular tachycardia). The study was approved by the local ethics committee and all patients gave their informed consent to participate.
Ablation workflowAblation was performed under local anesthesia, without systemic injection of drugs, in order to avoid masking PVCs. Sedation was minimized and delivered only in case of pain during ablation, using midazolam and fentanyl, as necessary. All the ablations were performed using the CARTO 3 electrophysiology mapping system (Biosense Webster, Diamond Bar, CA, USA). Access was obtained via the right femoral vein and/or artery depending on the site of origin of the PVC. Heparin was infused before any left ventricular access, and heparin dosing and activated clotting times were recorded with a target activated clotting time of 300-350 s. First, a spontaneous PVC template was generated for every clinical PVC. In cases of infrequent PVCs, isoproterenol infusion was started to obtain a reliable template. Detailed left and/or right ventricular geometry was then obtained to facilitate catheter positioning. Lastly, pacing through the ablation catheter was performed at multiple sites at a fixed pacing rate equal or close to the PVC coupling interval for patients with fixed or variable coupling intervals, respectively. Output of pace-mapping was set at 2 V/1 ms, and increased if needed when the ventricle could not be captured. Paced QRS morphologies were recorded in the electroanatomic mapping system. For each clinical PVC, a PaSo® map was generated to determine the zone with the closest matching morphology, where additional pacing morphologies were recorded. In cases with frequent PVCs, activation points were recorded using a PentaRay® catheter (Biosense Webster, Diamond Bar, CA, USA) at the suspected site of origin determined by the PaSo® map. Ablation was performed using a 3.5 mm tip, open-irrigated bidirectional Thermocool® SmartTouch® SF catheter (Biosense Webster, Diamond Bar, CA, USA). Applications of radiofrequency energy were titrated to a power of 30-40 W, and energy delivered for 60-120 s depending on the site of origin of the PVC. Ablation was considered successful if PVCs did not recur after a waiting period of 60 min, either spontaneously or after isoproterenol infusion.
Follow-upAntiarrhythmic medications were discontinued after an effective ablation procedure. Patients were seen in follow-up at 3-6 months post-ablation for a clinical assessment, an electrocardiogram (ECG) and a 24-h Holter recording. Subsequently, patients were seen on an as-needed basis. Long-term success was defined as 80% or greater reduction in PVC burden.
Statistical analysisNormally distributed variables were expressed as means ± standard deviation and compared using the Student's t test. Non-normally distributed variables were expressed as median and interquartile range (IQR) and compared using the Mann-Whitney U test. Categorical variables were expressed as counts and percentages and were compared using the chi-square test or Fisher's exact test when needed. A p-value <0.05 was considered statistically significant. The analyses were performed with the SPSS statistical package, version 11.0 (SPSS Inc., Chicago, IL).
ResultsStudy populationDuring the study period, 88 patients were referred for PVC ablation. Of these, 21 (23.8%) had multifocal PVCs and were included in the study. The characteristics of the study population are described in Table 1. Patients were mainly men and age was 62.7±14.8 years. Six (26.1%) patients had ischemic cardiomyopathy. The indication for PVC ablation was suspected PVC-induced left ventricular dysfunction/impairment, symptomatic PVCs or decreased biventricular pacing in 14 (66.7%), six (28.6%) and one (4.7%) patients, respectively. LVEF was ≥50% in only six (28.6%) patients, those with symptomatic PVCs, while the others had left ventricular dysfunction. The PVC burden was 24.4±10.4% (Table 2). Example ECGs of patients with multifocal PVCs included in the study (patients 19 and 21) are shown in Figure 1.
Clinical characteristics of the study population.
| Patient | Age, years | PVC burden | Cardiomyopathy | LVEF, % | BMI, kg/m2 | AF |
|---|---|---|---|---|---|---|
| 1 | 45 | 25% | Ischemic | 32 | 27.4 | 0 |
| 2 | 68 | 12% | Ischemic | 30 | 27.2 | 0 |
| 3 | 82 | 11% | Ischemic | 29 | 27.7 | 0 |
| 4 | 78 | 26% | Suspected PVCIC | 25 | 29.2 | 0 |
| 5 | 79 | 30% | Ischemic | 35 | 30.4 | Permanent |
| 6 | 51 | 41% | Suspected PVCIC | 42 | 25.2 | 0 |
| 7 | 80 | 45% | Ischemic | 15 | 24.7 | Permanent |
| 8 | 75 | 31.6% | Suspected PVCIC | 38 | 28.0 | 0 |
| 9 | 76 | 18% | 0 | 65 | 31.6 | 0 |
| 10 | 31 | 6% | 0 | 68 | 18.3 | 0 |
| 11 | 51 | 30% | Ischemic | 25 | 37.4 | 0 |
| 12 | 73 | 26% | 0 | 50 | 30.2 | 0 |
| 13 | 57 | 17% | Ischemic | 54 | 25.3 | 0 |
| 14 | 73 | 29% | Suspected PVCIC | 45 | 32.9 | 0 |
| 15 | 58 | 30% | Suspected PVCIC | 15 | 26.0 | 0 |
| 16 | 45 | 39% | Suspected PVCIC | 32 | 34.0 | 0 |
| 17 | 71 | 20% | 0 | 60 | 25.0 | 0 |
| 18 | 45 | 10% | 0 | 60 | 26.5 | 0 |
| 19 | 61 | 28% | Suspected PVCIC | 45 | 25.7 | 0 |
| 20 | 67 | 22.8% | Decrease in biventricular pacing | 44 | 28.7 | Paroxysmal |
| 21 | 48 | 15% | Suspected PVCIC | 35 | 22.9 | 0 |
AF: atrial fibrillation; BMI: body mass index; LVEF: left ventricular ejection fraction; PVC: premature ventricular contraction; PVCIC: premature ventricular contraction-induced cardiomyopathy.
Procedural characteristics of the study population.
| Patient | No. of pacing sites | PVCs | Best PaSo | No. of LAT sites | LAT, ms | Location of PVC | RF duration, min | Procedure duration, min | Fluoroscopy duration, min |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 99 | PVC 1 | 97.1% | 550 | 32 | Mid inferior LV | 21.2 | 240 | 15.1 |
| PVC 2 | 97.2% | 9 | 36 | Basal inferoseptal LV | |||||
| PVC 3 | 99.1% | 0 | NA | Apical lateral LV | |||||
| 2 | 142 | PVC 1 | 98.2% | 0 | NA | Basal inferolateral LV | 15.7 | 240 | 8.5 |
| PVC 2 | 95.4% | 0 | NA | Mid inferior LV | |||||
| PVC 3 | 99.1% | 0 | NA | Basal anterolateral LV | |||||
| 3 | 93 | PVC 1 | 97.8% | 2 | 22 | Apical lateral LV | 10.2 | 180 | 10.1 |
| PVC 2 | 98.4% | 0 | NA | Mid anterolateral LV | |||||
| 4 | 21 | PVC 1 | 96.2% | 373 | 29 | Inferoseptal RVOT | 16.8 | 180 | 13.2 |
| PVC 2 | 98.5% | 125 | 44 | Antero-lateral PM | |||||
| 5 | 61 | PVC 1 | 97.6% | 10 | 29 | Inferoseptal RVOT | 14.2 | 120 | 7.3 |
| PVC 2 | 96.6% | 5 | 30 | Apical anterior LV | |||||
| PVC 3 | 99.0% | 5 | 30 | Laterobasal RV | |||||
| 6 | 70 | PVC 1 | 98.3% | 543 | 37 | Postero-median PM | 42.8 | 240 | 11.5 |
| PVC 2 | 98.9% | 0 | NA | Basal anterior LV | |||||
| 7 | 106 | PVC 1 | 99.1% | 229 | 25 | Mid inferolateral LV | 9.6 | 140 | 15.5 |
| PVC 2 | 93.0% | 2 | 18 | Basal anterior LV | |||||
| 8 | 42 | PVC 1 | 97.3% | 218 | 18 | Basal anterior LV | 3.5 | 150 | 4.4 |
| PVC 2 | 98.9% | 7 | 37 | Basal inferoseptal LV | |||||
| 9 | 137 | PVC 1 | 97.6% | 20 | 25 | Mid inferior LV | 14.4 | 150 | 7.1 |
| PVC 2 | 94.0% | 0 | NA | Mid anterolateral LV | |||||
| 10 | 104 | PVC 1 | 96.5% | 0 | NA | Inferoseptal RVOT | 2.5 | 180 | 6.4 |
| PVC 2 | 86.3% | 0 | NA | Basal inferolateral RV | |||||
| 11 | 135 | PVC 1 | 94.4% | 0 | NA | Mid anteroseptal LV | 14.7 | 180 | 6.4 |
| PVC 2 | 97.3% | 0 | NA | Mid inferior LV | |||||
| PVC 3 | 96.1% | 0 | NA | Mid inferoseptal LV | |||||
| 12 | 99 | PVC 1 | 96.3% | 71 | 33 | Basal anterior LV | 20.0 | 360 | 21.4 |
| PVC 2 | 96.7% | 15 | 45 | Basal anteroseptal LV | |||||
| 13 | 110 | PVC 1 | 97.8% | 490 | 21 | Anterior RVOT | 33.2 | 240 | 14.7 |
| PVC 2 | 98.0% | 316 | 23 | Mid inferolateral RV | |||||
| 14 | 33 | PVC 1 | 92.0% | 264 | 25 | Basal septal RV | 8.7 | 180 | 8.7 |
| PVC 2 | 96.2% | 195 | 18 | Septal RVOT | |||||
| 15 | 65 | PVC 1 | 94.6% | 663 | 34 | Basal inferoseptal LV | 7.0 | 120 | 7.2 |
| PVC 2 | 93.7% | 27 | 26 | Basal anterior LV | |||||
| 16 | 40 | PVC 1 | 97.7% | 1376 | 29 | Antero-lateral PM | 24.0 | 180 | 4.1 |
| PVC 2 | 95.9% | 119 | 27 | Basal septal RV | |||||
| 17 | 22 | PVC 1 | 99.1% | 363 | 70 | Left coronary cusp | 9.2 | 240 | 27.7 |
| PVC 2 | 92.0% | 129 | 18 | Basal inferoseptal LV | |||||
| PVC 3 | 98.1% | 91 | 18 | Basal anterolateral LV | |||||
| 18 | 27 | PVC 1 | 96.2% | 455 | 25 | Septal RVOT | 13.9 | 180 | 12.3 |
| PVC 2 | 98.8% | 101 | 25 | Anterior RVOT | |||||
| 19 | 21 | PVC 1 | 96.6% | 488 | 60 | Lateral RVOT | 4.5 | 120 | 17.3 |
| PVC 2 | 80.7% | 29 | 5 | Basal anterior LV | |||||
| 20 | 90 | PVC 1 | 97.4% | 70 | 21 | Mid inferoseptal LV | 31.3 | 180 | 6.2 |
| PVC 2 | 98.8% | 3 | 21 | Basal inferior LV | |||||
| 21 | 35 | PVC 1 | 95.6% | 231 | 39 | Antero-lateral PM | 9.2 | 240 | 27.7 |
| PVC 2 | 97.2% | 0 | NA | Postero-lateral PM |
LAT: local activation time; LV: left ventricle; NA: not available; PM: papillary muscle; PVC: premature ventricular contraction; RF: radiofrequency; RV: right ventricle; RVOT: right ventricular outflow tract.
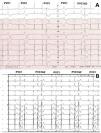
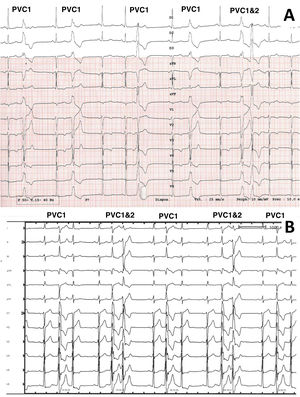
12-lead electrocardiographic traces of patients included in the study, patients 19 and 21. For patient 19 (A), PVC1 and PVC2 were coming from the lateral right ventricular outflow tract and the anterobasal left ventricle, respectively. For patient 21 (panel B), PVC 1 and PVC2 were coming from the anterolateral and posteromedial papillary muscles, respectively.
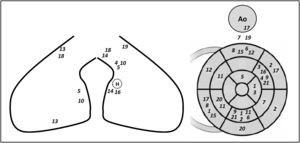
A total of 47 PVCs were targeted with ablation in the 21 patients included. Five patients presented three PVC morphologies, while the other 16 patients had two PVCs morphologies. multifocal PVCs were all located in the same ventricle in 17 patients (left ventricle [LV]: 13 patients, right ventricle [RV]: four patients) or in both ventricles in four patients. A schematic representation of the location of the multifocal PVCs mapped for every patient included is shown in Figure 2.
Schematic representation of the location of multifocal premature ventricular contractions for every patient included. Each number represents a PVC of a patient included (from 1 to 21). The left panel represents the right ventricle (lateral and septal walls on the left and middle panels, respectively) H: His bundle; Ao: aorta.
After constructing the ventricular geometry, detailed pace-mapping was performed by pacing an average of 73.5±41.6 different locations in the LV and/or RV. The median time taken to obtain all pacing points was 30.0 min (IQR 15.0-45.0). The best matching generated was 97.2% (IQR 95.9%-98.3%) similar to the clinical PVC targeted. Typical examples of maps generated in patients with multiple PVCs (patients 1, 2 and 11) are shown in Figures 3–5. As shown, after pacing the cavity, focusing on the supposed location of the PVCs’ site of origin, the system can generate a specific map for each PVC morphology, spotting the precise zone of the ventricle where pacing generates a QRS resembling the targeted extra beat.
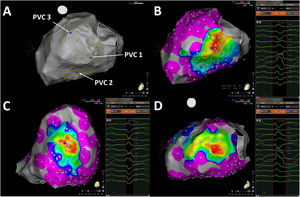
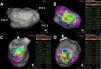
Pace-mapping map generated in patient 1. A total of 99 pacing points were acquired (panel A). The correlation percentage and a color-coded map were automatically generated for each premature ventricular contraction (PVC): 97.1% (mid-inferior left ventricle [LV], panel B), 97.2% (basal inferoseptal LV, panel C) and 99.1% (apical lateral LV, panel D) for PVC1, PVC2 and PVC3, respectively. Local activation time points could only be obtained for PVC1 and PVC2.
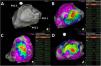
Pace-mapping map generated in patient 2. A total of 142 pacing points were acquired (A). The correlation percentage and a color-coded map were automatically generated for each premature ventricular contraction (PVC): (B) 98.2% (basal inferolateral LV), (C) 95.4% (mid inferior LV) and (D) 99.1% (basal anterolateral LV) for PVC1, PVC2 and PVC3, respectively. No local activation time points could be obtained in this patient due to the paucity of PVCs during the procedure.
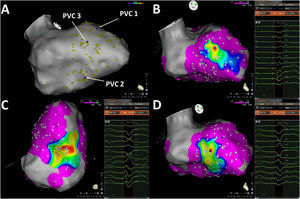
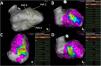
Pace-mapping map generated in patient 11. A total of 135 pacing points were acquired (A). The correlation percentage and a color-coded map were automatically generated for each premature ventricular contraction (PVC): (B) 94.4% (mid anteroseptal LV), (C) 97.3% (mid inferior LV) and (D) 96.1% (mid inferoseptal LV) for PVC1, PVC2 and PVC3, respectively. No local activation time points could be obtained in this patient due to the paucity of PVCs during the procedure.
Once the precise location of the PVC was determined through analysis of pace-mapping, activation points were taken, if possible, in the area surrounding the best pace-match. For the prevalent PVC (PVC1 in Table 2), a median of 231.0 (IQR 17.5-488.5) activation points were obtained, using a PentaRay, although no activation points could be obtained for three patients (Table 2: patients 2, 10 and 11). Ablation in these three patients was based solely on pace-mapping. A significantly lower number of activation points were obtained for the less prevalent PVC2 (median of seven [IQR 0.0-105.5] activation points, p=0.002 compared to PVC1) and ablation was based on pace-mapping for only seven patients, patients 2, 3, 6, 9, 10, 11 and 21 (Table 2) since no activation points were obtained for their PVC2. Similarly, ablation of PVC3 for patients with three different PVC morphologies was based solely on pace-mapping in three out of five patients. Thus, in summary, ablation was based only on pace-mapping in 13/47 (27.6%) of the patients, and ≤10 local activation time (LAT) points were obtained in eight more PVCs (17.0%).
Ablation of premature ventricular contractions and follow-upA total of 17.2 (IQR 9.4-21.3) min of radiofrequency were delivered during the procedure. Procedure and fluoroscopy time were 180 (IQR 150-240) and 10.5±6.3 min, respectively. No procedure-related complications occurred during ablation or hospitalization. Complete acute procedural success, defined as the elimination of all PVCs at least 60 min after ablation, was obtained in 14 (66.7%) patients. One PVC morphology was deliberately not ablated in five patients (23.8%) due to its proximity to the conduction system (patients 14, 16 and 17) or to its location in the LV apex (patients 7 and 19). The other two patients had acute failures.
As previously stated, ablation was solely based on pace-mapping for three patients (patients 2, 10 and 11) (Table 2). The procedure was successful in these patients, and their PVC burden decreased from 12%, 6% and 30% to 1%, 1% and 2%, respectively.
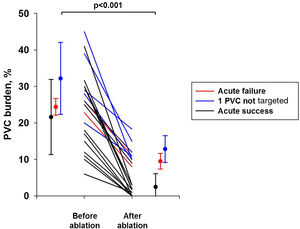
After 12.3±9.4 months of follow-up, overall PVC burden decreased from 24.4±10.4% to 5.6±5.0% (p<0.001). As shown in Figure 6, two patients with acute procedural success had PVC recurrence (patients 1 and 12). Interestingly, patients with acute procedural failure with some PVCs deliberately not targeted during the procedure also experienced a significant decrease in PVC burden. Overall, PVC burden decreased from 21.6% to 2.5% (88% reduction), from 24.4% to 9.5% (61% reduction) and from 32.2% to 12.9% (60% reduction) in patients with acute success, acute failure, and some PVCs deliberately not targeted, respectively.
Among the 14 patients with suspected PVC-induced left ventricular dysfunction/impairment, LVEF did not change in four patients (from 30.8% to 28.4%), but improved in the other 10 (from 34.1% to 53.0%, p<0.001), confirming the role of PVCs in their left ventricular dysfunction.
DiscussionMain resultsThe main results of this study are: (1) one in five patients referred for PVC ablation may present multifocal PVCs; (2) detailed morphology-matching mapping using dedicated software can be performed to identify the site of origin of each single PVC, and successful ablation performed at these sites, even if LATs cannot be obtained; and (3) this strategy results in a fairly good long-term success rate.
Multifocal premature ventricular contractionsAblation of PVCs can be considered in symptomatic patients or in those with LVEF impairment suspected to be partially or totally due to a high PVC burden (PVC-induced cardiomyopathy).1 In most cases a single PVC morphology is observed, and ablation focuses on the site of origin, in either the LV or the RV. Success rates as high as 80% have been reported with experienced teams.5 Data regarding the prevalence of patients with multifocal PVCs are scarce. In a large multicenter retrospective study including 1185 patients who underwent catheter ablation for idiopathic PVCs, Latchamsetty et al. reported that 18% of patients had multifocal PVCs.6 Patients with multifocal PVCs had longer procedure (100 min longer, p<0.001), fluoroscopy (21 min longer, p<0.001) and RF times (3 min longer, p<0.001) compared to patients with a single PVC focus. The acute procedural success rate, defined as elimination of the targeted PVC at least 30 min after ablation, was lower than for single PVCs (75% vs. 85%). Long-term success without antiarrhythmic drugs, defined as an 80% reduction in PVC burden, was even lower at 62%. Interestingly, the presence of multifocal PVCs was an independent predictor of acute procedural failure, increasing the risk of failure by 11% for each additional PVC morphology (odds ratio 0.89; 95% confidence interval 0.82-0.98, p=0.01). Consequently, as stated by the authors, not only the site of origin, but also the number of different PVCs needs to be considered when counseling patients for the ablation procedure.
Recently, Sheldon et al. specifically studied the clinical characteristics and outcomes of patients with multifocal PVCs.7 In a subset of 100 patients with no structural heart disease (LVEF 57%±9), 31 had multifocal PVCs. A cutoff of ≥156 non-predominant PVCs over 24 hours best separated successful from unsuccessful procedures. As previously demonstrated, patients with a single PVC focus had a better procedural success rate. Interestingly, patients with multifocal PVCs had significantly more epicardial foci and a trend towards more papillary muscle origin, i.e. locations known to be associated with more complex procedures, and consequently lower success rates. Procedural failures were shown to be the consequence of re-emergence of the previously targeted predominant PVC, and not of an increase in previously non-predominant or undocumented PVCs. Consequently, the authors stated that procedural success can sometimes be obtained even if not all PVCs are successfully ablated.
Ablation strategyAblation of PVCs can be performed targeting either the earliest activation site, by means of an activation map, or the site with the closest morphology match, by means of a pace-matching map. When there is a high PVC burden at the time of the procedure, activation mapping is usually performed. The spatial resolution of an activation map is considered to be superior to that of pace-mapping, as shown in right ventricular outflow tract PVCs.8–10 However, some teams have demonstrated contrary results.11 To validate the site of origin detected by the activation map, or in cases in which few PVCs are observed at the onset of the procedure, which is known to predict ablation failure,4 pace-mapping points can be acquired. The required specifications for an optimal pacing map are optimal contact with the tissue, a low pacing output to capture only the local myocardium through the smallest possible virtual electrode, and a pacing cycle similar or close to the PVC coupling interval in order to reproduce possible functional conduction blocks.12 Pace-matching ≥89% was shown to be a good predictor of successful ablation, with sensitivity and specificity of 95% and 80%, respectively.13 The technique used to process PVC matching is also of importance, since automated template matching has been shown to be superior to visual matching.13
Technological advances like PaSo® software help greatly to obtain a detailed and accurate pace-matching map.14 After a reference on the body surface lead is selected, a clinical PVC pattern is acquired. Thereafter, pacing morphologies are acquired and compared to the recorded PVC pattern. An overall correlation percentage is calculated by averaging the specific correlation percentage of each ECG lead. If multifocal PVCs are present, a pattern bank including all PVC morphologies can be obtained to generate a detailed PaSo® map in order to spot the site of origin of each ectopic beat, as performed in our study. If the PVC burden was sufficient, LATs were recorded to confirm the PVC origin, while ablation relied solely on pace-matching if no LAT points could be acquired. As noted above, paucity of PVCs at the beginning of an ablation procedure is associated with an almost seven-fold decrease in ablation success rate, regardless of the site of origin,4 an observation that highlights the need for an effective alternate technique to achieve successful ablation in cases of infrequent PVCs, as observed with morphology-matching software. In a small retrospective study, Moak et al. showed that a fairly good ablation success rate can be obtained using pace-matching only, especially when correlation coefficients over 92% are obtained.15
LimitationsThis is a single-center study, in a limited number of patients, of the use of PaSo® software to ablate multifocal PVCs. Its results should be confirmed in larger multicenter studies. To exclude the possibility that non-predominant PVCs represented just a different exit site from the predominant PVC, multifocal PVCs were defined as PVCs coming from different myocardial regions. However, since patients with structural heart disease were included, some with prior myocardial infarction and slow conduction areas, we cannot exclude that the same PVC site of origin could result in two PVC morphologies, exiting from adjacent ventricular regions.
In this paper, we aimed to determine the efficacy of an automated pace-mapping-based ablation strategy for multifocal PVCs. However, as shown in Table 2, activation points could be obtained for most of the PVCs mapped and only three patients had a full procedure based solely on pace-mapping. Thus, this does not permit adequate assessment of the success of pace-mapping alone versus pace-mapping in combination with activation mapping, and further studies would be needed to determine whether pace-mapping alone is sufficient for a successful procedure.
Lastly, the exact burden of every predominant and non-predominant PVC was not ascertained in this study prior to and after ablation. Thus, the recurrence of PVC in patients with procedural failure could not be precisely determined.
ConclusionMultifocal PVCs can frequently be observed in patients referred for ablation of ventricular ectopic beats. Detailed quantitative morphology-matching software can be used to obtain a detailed map identifying the site of origin of each single PVC, and successful ablation performed at these sites, even if LATs cannot be obtained due to the paucity of ectopic beats.
Conflicts of interestRPM has received lecture fees from Biosense-Webster.
The authors thank Lucas De Courreges for his invaluable electrophysiological advice.




![Pace-mapping map generated in patient 1. A total of 99 pacing points were acquired (panel A). The correlation percentage and a color-coded map were automatically generated for each premature ventricular contraction (PVC): 97.1% (mid-inferior left ventricle [LV], panel B), 97.2% (basal inferoseptal LV, panel C) and 99.1% (apical lateral LV, panel D) for PVC1, PVC2 and PVC3, respectively. Local activation time points could only be obtained for PVC1 and PVC2. Pace-mapping map generated in patient 1. A total of 99 pacing points were acquired (panel A). The correlation percentage and a color-coded map were automatically generated for each premature ventricular contraction (PVC): 97.1% (mid-inferior left ventricle [LV], panel B), 97.2% (basal inferoseptal LV, panel C) and 99.1% (apical lateral LV, panel D) for PVC1, PVC2 and PVC3, respectively. Local activation time points could only be obtained for PVC1 and PVC2.](https://static.elsevier.es/multimedia/08702551/0000004100000008/v2_202209110531/S087025512200155X/v2_202209110531/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)