Acute coronary syndromes (ACS) mostly occur in patients with traditional risk factors. Especially in young adults without major cardiovascular (CV) risk factors, one of the less common causes of ACS is myeloproliferative neoplasms (MPNs).
MethodsWe retrospectively collected data on 11 consecutive patients (nine men, two women, mean age 40.18±8.4 years) with a diagnosis of MPN who presented with ACS. The demographic characteristics of the study population, type of MPN, clinical manifestations, location of myocardial infarction (MI), coronary angiography findings, complete blood count and other related findings, and treatment strategy before and after diagnosis were analyzed.
ResultsSix patients were diagnosed with polycythemia vera, four with essential thrombocytosis and one with primary myelofibrosis. A JAK2 mutation was found in nine patients. Mean time to diagnosis of MPN was 2.81 years after presenting ACS and mean age at first MI was 32.9±6 years. Six patients had no major CV risk factors. Ten patients had anterior MI and one had inferior MI. After initiation of specific treatment for MPN, no recurrent thrombotic events were observed in a mean follow-up of 4±2.44 years.
ConclusionsIn young adults presenting with ACS, MPNs should be considered, especially in the absence of atherosclerotic coronary artery lesions. It is also important to pay attention to blood cell count abnormalities seen in intracoronary thrombotic events. Early diagnosis and treatment of MPNs is essential to prevent recurrence of thrombotic events and may reduce mortality and morbidity related to thrombotic complications.
As síndromes coronárias agudas (SCA) ocorrem sobretudo em doentes com fatores de risco tradicionais. No caso particular dos adultos jovens sem fatores de risco cardiovasculares (CV) major, as neoplasias mieloproliferativas (NMP) são uma das causas raras de SCA.
MétodosRecolhemos dados retrospetivos de 11 doentes consecutivos com SCA (9 homens, 2 mulheres, de idade média de 40,18 ± 8,4) com o diagnóstico de NMP. Foram analisados os dados sobre as características demográficas da população do estudo, o tipo de NMP, as manifestações clínicas, a localização do enfarte do miocárdio (EM), os achados da angiografia coronária (AC), hemograma, outros achados relacionados, a estratégia de tratamento antes e após o diagnóstico.
ResultadosSeis doentes foram diagnosticados com policitemia vera (PV), quatro doentes com trombocitemia essencial (TE) e um doente com mielofibrose primária (MFP). A mutação JAK-2 foi encontrada em nove doentes. Após apresentação da SCA, o tempo médio de diagnóstico da NMP foi de 2,81 anos. A idade média dos doentes foi de 32,9 ± 6 quando sofreram pela primeira vez um enfarte do miocárdio. Seis doentes não manifestaram fatores de risco CV major. Dez doentes tiveram EM anterior, um doente teve EM inferior. Após o início do tratamento específico da NMP, não foram observados eventos trombóticos recorrentes com um seguimento médio de 4 ± 2,44 anos.
ConclusõesNos adultos jovens com SCA, deve ser considerada a NMP, especialmente na ausência de lesões ateroscleróticas das artérias coronárias. É igualmente importante estar atento às anomalias no hemograma encontradas nos eventos trombóticos intracoronários. Um diagnóstico e um tratamento precoces da NMP são essenciais para prevenir a recidiva de eventos trombóticos e podem reduzir a mortalidade e a morbilidade relacionadas com complicações trombóticas.
Acute coronary syndrome (ACS) mostly occurs in patients with traditional risk factors such as hypertension, diabetes, dyslipidemia, smoking or family history. Especially in young adults without major cardiovascular (CV) risk factors, one of the less common causes of ACS is myeloproliferative neoplasms (MPNs). These are a heterogeneous group of diseases characterized by overproduction of pluripotent hematopoietic stem cells in the bone marrow. MPNs include polycythemia vera (PV), essential thrombocytosis (ET), primary myelofibrosis (PMF) and chronic myeloid leukemia.1
Thrombotic complications are common and increase mortality and morbidity in these patients. Arterial thrombosis is more common than venous thrombosis in both PV and ET. Generally large arteries, especially in the cardiovascular and cerebrovascular systems, are affected in PV, while microcirculatory system involvement is typical in ET. Multiple factors contribute to the development of thrombosis, including increased cell mass, activation and number of platelets and leukocytes and their interactions, and inflammatory response of vascular cells to cytokines and other mediators released by proliferative blood cells.2,3 The presence of the V617F mutation in JAK2 is one of the strongest risk factors for thrombotic complications, with a two-fold increased risk.4,5 The exact incidence of thrombotic events in patients with MPN is hard to ascertain. In several published series, the rate of CV complications related to MPNs ranged from 4% to 21%.6
The aim of this retrospective analysis is to assess the demographic, clinical and laboratory features of patients with MPN presenting with ACS.
MethodsWe retrospectively collected data on 11 consecutive patients followed in the hematology and cardiology departments of our hospital with a diagnosis of MPN who presented with ACS. MPNs were defined according to the 2016 World Health Organization diagnostic criteria, which include blood cell count (CBC), peripheral blood smear, genetic investigations (JAK2 V617F mutation, JAK2 exon 12 mutations, calreticulin mutations, and MPL mutations), bone marrow aspiration and biopsy, flow cytometry and clinical features.7 We analyzed the demographic characteristics of the study population, type of MPN, clinical manifestations, location of myocardial infarction (MI), coronary angiography findings, CBC, and other related findings such as jugular vein thrombosis, peripheral arterial thrombosis and hepatosplenomegaly. Treatment strategy before and after diagnosis were also analyzed.
The study protocol was approved by the local ethics committee of our institution, and detailed written informed consent was obtained from each patient. The study was in accordance with the Declaration of Helsinki.
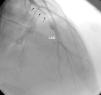
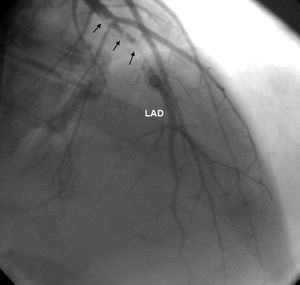
ResultsEleven patients who presented with ACS and were diagnosed with MPNs were studied (nine men, two women, mean age 40.18±8.4 years). Six patients had no major CV risk factors; only one had two risk factors, while the rest had one. The patients’ mean age was 32.9±6 years when they first suffered MI. Case 1 also had concomitant jugular vein thrombosis, case 2 had portal vein thrombosis and case 11 had peripheral arterial thrombosis. Splenomegaly was seen in five patients and hepatosplenomegaly in two. Ten patients had anterior MI and one had inferior MI. In two patients (cases 2 and 4) MI was complicated by cardiac arrest. On coronary angiography, six patients had left anterior descending (LAD) lesions (Figure 1 and Supplementary Video 1 represent case 4), two patients had LAD and concomitant diagonal lesions, one patient had LAD and circumflex artery lesions, one patient had a right coronary artery lesion and one patient's angiography was normal. All lesions were thrombotic, causing total or sub-total occlusion, and coronary arteries other than the infarct-related arteries were normal. Nine patients underwent percutaneous coronary intervention and two patients underwent coronary artery bypass grafting due to dissection of the LAD (Table 1).
Demographic and clinical characteristics of the study group.
| Case no./age, years/gender | CV risk factors | First presentation | Coronary angiogram | Other findings | Treatment after MI |
|---|---|---|---|---|---|
| 1/54/M | None | Anterior MI | LAD | SM, JVT | Aspirin |
| 2/47/F | None | Inferior MI, cardiac arrest | RCA | SM, PVT | Aspirin, nebivolol, ticagrelor |
| 3/49/M | Hypertension, hyperlipidemia | Anterior MI | LAD, CX | SM | Aspirin, nebivolol, atorvastatin |
| 4/27/M | None | Anterior MI, cardiac arrest | LAD | None | Aspirin, clopidogrel |
| 5/45/F | Smoking | Anterior MI | LAD | SM | Aspirin, clopidogrel |
| 6/32/M | None | Anterior MI | LAD, D1 | None | Aspirin |
| 7/31/M | None | Anterior MI | LAD | None | Aspirin, clopidogrel, bisoprolol |
| 8/46/M | Smoking | Anterior MI | LAD, D1 | HSM | Aspirin |
| 9/43/M | None | Anterior MI | LAD | HSM | Clopidogrel, statin |
| 10/32/M | Smoking | Anterior MI | LAD | None | Aspirin, ramipril, carvedilol |
| 11/39/M | Smoking | Anterior MI | Normal | SM, PAT | Aspirin, bisoprolol, spironolactone |
CV: cardiovascular; CX: circumflex artery; D1: first diagonal artery; F: female; HSM: hepatosplenomegaly; JVT: jugular vein thrombosis; LAD: left anterior descending artery; M: male; MI: myocardial infarction; PAT: peripheral arterial thrombosis; PVT: portal vein thrombosis; RCA: right coronary artery; SM: splenomegaly.
Six patients in the study group were diagnosed with PV, four with ET and one with PMF. Mean time to diagnosis of MPN after MI was 2.81 years (Table 2).
Hematologic features and laboratory findings at the time of myocardial infarction.
| Case no./age, years/gender | Diagnosis | Hb, g/dl | HCT, % | Platelets, ×109/l | Leukocytes, ×109/l | JAK2 V617 mutation | Current treatment |
|---|---|---|---|---|---|---|---|
| 1/54/M | PMF | 11.2 | 37.6 | 249 | 28.7 | Yes | PT+HU+ruxolitinib |
| 2/47/F | PV | 13.5 | 41 | 385 | 9.25 | Yes | PT+HU+warfarin |
| 3/49/M | PV | 15 | 45 | 791 | 8.27 | Yes | PT+HU+warfarin |
| 4/27/M | ET | 13.6 | 39.7 | 771 | 13.4 | Yes | Aspirin+IFN+warfarin |
| 5/45/F | ET | 14.3 | 42 | 655 | 9.75 | Yes | Aspirin+HU |
| 6/32/M | ET | 15.6 | 46.8 | 498 | 16.1 | No | Aspirin+IFN+warfarin |
| 7/31/M | PV | 17.3 | 52 | 455 | 8.3 | Yes | PT+HU |
| 8/46/M | PV | 19.5 | 58.2 | 366 | 15.4 | Yes | PT+HU |
| 9/43/M | ET | 15.4 | 46 | 850 | 10.7 | Yes | PT+HU+warfarin |
| 10/32/M | PV | 17.7 | 53.6 | 774 | 25.1 | No | PT+HU |
| 11/39/M | PV | 16.3 | 45 | 444 | 9.1 | Yes | PT+IFN+warfarin |
ET: essential thrombocytosis; F: female; Hb: hemoglobin; HCT: hematocrit; HU: hydroxyurea; IFN: interferon alfa-2a; M: male; PMF: primary myelofibrosis; PT: previous treatment before MPN diagnosis; PV: polycythemia vera.
All patients had been receiving anti-ischemic medication when they were diagnosed with MPN. The specific therapy chosen for MPN was primarily cytoreductive therapy including hydroxycarbamide (hydroxyurea), ruxolitinib and interferon alfa-2a. Phelobotomy was performed in patients with PV who had hematocrit >45%. Anticoagulant therapy with warfarin was used to treat thrombosis and to prevent recurrence of thrombotic events. Warfarin could not be used in four patients due to hemorrhagic complications or patient refusal. After initiation of specific treatment for MPN, no recurrent thrombotic events were observed in a mean follow-up of 4±2.44 years.
DiscussionAll patients in the study group presented with MI related to MPNs. Acute coronary syndrome can be the first manifestation of MPN. These two conditions most frequently affect middle-aged and elderly populations. However, cases have been reported in younger populations.8 In contrast to the literature, our patients were under 40 years of age when they first suffered MI. One of the most intriguing findings in our study population was that only three patients had been diagnosed with MPN immediately after MI. For the other patients, the diagnosis of MPN was made within 1-10 years.
Blood cell count abnormalities like reactive thrombocytosis and leukocytosis may be seen in acute MI as an inflammatory response,9,10 and may lead to missed or delayed diagnosis of MPN. CBC tests should therefore be repeated after some time. If there is no change in elevated CBC parameters, further hematologic assessment should be considered. Careful abdominal examination including, if necessary, abdominal ultrasound is important to detect hepatosplenomegaly, which can indicate thrombocytosis, leukocytosis or polycythemia in these patients.
Additional CV risk factors may increase the risk of thrombotic complications in MPNs. In our analysis, nearly half of the patients had additional risk factors. In addition, patients with JAK2 mutations have a high incidence of thrombotic events. A JAK2 mutation is present in nearly 95% of patients with PV and in about 50% of those with ET and PMF.11,12 In our study all except two patients had the JAK2 V617 mutation. Diagnosis in these two patients was made by bone marrow aspiration and biopsy findings.
Data on which coronary arteries are most affected in ACS related to MPNs are scarce; in our study LAD lesions and anterior MI were predominant.
To the best of our knowledge, although there are several case reports in the literature,13–15 our study is the first retrospective study of patients experiencing ACS as a first manifestation of MPNs.
In conclusion, in young adults presenting with ACS, other disorders that can precipitate thrombotic events, like MPNs, should be considered, especially in the absence of atherosclerotic coronary artery lesions. It is also worth reminding the interventional cardiologist of the importance of blood cell count abnormalities seen in patients suffering intracoronary thrombotic events. Early diagnosis and treatment of MPNs is essential to prevent recurrence of thrombotic events and may reduce mortality and morbidity related to thrombotic complications.
FundingThe study did not receive any type of financial support.
This study was carried out at Florence Nightingale Hospital Department of Cardiology.
Conflicts of interestThe authors have no conflicts of interest to declare.