Ventricular arrhythmias are caused by scar tissue in patients with ischemic dilated cardiomyopathy. The gold standard imaging technique for detecting scar tissue is magnetic resonance imaging (MRI). However, MRI is not feasible for use as a screening test, and also cannot be used in patients who have received an implantable cardioverter-defibrillator (ICD). In this study, we aimed to assess the association between levels of galectin-3 (Gal-3), which is known to be secreted by scar tissue, and the history of ventricular arrhythmias in patients with ischemic dilated cardiomyopathy who received an ICD.
MethodsNineteen healthy controls and 32 patients who had previously undergone VVI-ICD implantation due to ischemic dilated cardiomyopathy were enrolled in the study. Patients were divided into three groups: the first group including patients who had received no ICD therapies, the second including patients with arrhythmia requiring therapies with no arrhythmia storm, and the third including patients who had arrhythmia storm. We assessed the association between Gal-3 levels and the history of ventricular arrhythmias in these patients.
ResultsGal-3 levels were significantly higher in the patient groups than in the control group (p<0.01). Gal-3 levels of patients with arrhythmias requiring ICD therapies were significantly higher than in patients with ICD not requiring therapies (p=0.02). They were also higher in patients with a history of arrhythmia storm than in patients without shocks (p=0.05). Receiver operating curve analysis showed with 84% sensitivity and 75% specificity that Gal-3 levels over 7 ng/ml indicated ventricular arrhythmia that required therapies.
ConclusionGal-3 may be used to further improve risk stratification in patients with ischemic cardiomyopathy who are more prone to developing life-threatening arrhythmias.
Em doentes com miocardiopatia isquémica dilatada as arritmias ventriculares são causadas por tecidos cicatriciais. O gold standard das técnicas de imagem na deteção de fibrose é a ressonância magnética (RM). No entanto, esta não é viável como teste de rastreio e também não pode ser utilizada em muitos doentes com cardioversor desfibrilhador implantável (CDI). Neste estudo, pretendemos avaliar a associação entre o nível de Galectina-3 (Gal-3), que é secretada por tecidos cicatriciais, e a história de arritmias ventriculares em doentes com miocardiopatia isquémica dilatada com CDI implantado.
MétodosDezanove casos controlo saudáveis e 32 doentes, previamente submetidos a implantação de CDI-VVI por miocardiopatia isquémica dilatada, foram incluídos. Os doentes foram divididos em três grupos. O primeiro grupo incluiu doentes que não se submeteram a tratamento. O segundo grupo incluiu doentes com arritmias que necessitaram de tratamento mas sem tempestade arritmica. O último grupo incluiu doentes com tempestades arrítmicas. Avaliámos a associação entre os níveis de Gal-3 e a história de arritmias ventriculares naqueles doentes.
ResultadosO nível de Gal-3 foi significativamente mais elevado no grupo de doentes do que no grupo controlo (<0,01). Os níveis de Gal-3 nos doentes com arritmias que necessitaram de tratamento com CDI foram significativamente mais elevados do que nos doentes com CDI que não necessitaram de tratamento (p: 0,02). Os níveis de Gal-3 foram mais elevados nos doentes com detempestade arrítmica do que nos doentes sem choques (p: 0,05). Verificámos nas análises ROC, com sensibilidade de 84% e com especificidade de 75%, que níveis de Gal-3 acima de 7 ng/mL são preditores de arritmia ventricular que requer tratamento.
ConclusãoA Gal-3 pode ser utilizada para melhorar a estratificação de risco em doentes com miocardiopatia isquémica dilatada, identificando os doentes com maior propensão a desenvolver arritmias ventriculares malignas.
Heart failure is one of the most important causes of mortality and morbidity and ischemic heart disease is the most common cause of heart failure.1,2 In the advanced stages of ischemic heart disease-related heart failure, the frequency of arrhythmic episodes increases. Ventricular arrhythmias can cause fatal outcomes in these patients.3,4 In order to reduce mortality, an implantable cardioverter-defibrillator (ICD) is recommended for selected patients, subject to certain indications. However, studies have shown that when these indications are taken into account, only one third of patients receive appropriate ICD therapies. Also, a high percentage of patients who do not fulfill these indications and do not have an ICD are resuscitated from sudden cardiac death. Therefore, additional parameters are needed in patients with ischemic cardiomyopathy to improve the indications and risk stratification.
Human studies have shown an independent relationship between myocardial fibrosis and arrhythmia episodes.5,6 Magnetic resonance imaging (MRI) studies have shown a relationship between myocardial fibrosis load and ventricular tachycardia episode frequency and prognosis.7,8 However, MRI is not suitable for use as a screening test. Also, the load and distribution of fibrosis after ICD implantation cannot be assessed using MRI.
Galectin-3 (Gal-3) is a beta-galactosid binding protein that has been shown to be associated with cardiac fibrosis9,10 and also with mortality in acute and chronic heart failure.11
In this study, we aimed to assess the association between ventricular fibrillation/ventricular tachycardia (VF/VT) and the quantity of left ventricular fibrotic tissue assessed using blood Gal-3 levels in ischemic cardiomyopathy patients with a VVI-ICD.
MethodsThis was a cross-sectional study including adults treated in the cardiac arrhythmia outpatient clinic of a university hospital between January and April 2017. Patients diagnosed with ischemic dilated cardiomyopathy and who underwent VVI-ICD implantation for primary or secondary prevention were included in the study after their informed consent was obtained. Patients who had had a myocardial infarction (MI) in the previous year, patients with renal insufficiency (creatinine clearance<60 ml/min/1.73 m2 calculated by the 4-variable Modification of Diet in Renal Disease or Cockcroft-Gault formulas), those with inappropriate shocks, or those with a VVI-ICD for non-ischemic dilated cardiomyopathy, arrhythmogenic right ventricular dysplasia (ARVD), Brugada syndrome, long or short QT syndrome, or hypertrophic cardiomyopathy, were excluded from the study.
Healthy volunteers with no known medical problems were selected from hospital staff as controls. Data on age, gender, type of MI, comorbidities (including diabetes, hypertension and chronic obstructive pulmonary disease), smoking and medications were recorded for all participants. ICD interrogation was performed in 32 patients and all arrhythmia episodes in the previous three years were recorded, as were patients’ medical histories from the outpatient clinic files. Arrhythmic events were analyzed retrospectively.
Blood samples to measure Gal-3 levels were taken from all participants between 8 a.m. and 9 a.m., after 8-12 hours fasting. Blood was placed in ethylenediaminetetraacetic acid tubes and centrifuged for 15 min at 3500 rpm and serum samples were obtained, which were stored at -80°C until the day of study. Plasma Gal-3 levels were measured by enzyme-linked immunosorbent assay (ARCHITECT i2000SR, Abbott Diagnostics, Abbott Park, IL, USA). The manufacturer's recommended limit was 4-114 ng/ml.
Treatments from intracardiac electrograms were reviewed from outpatient clinic records and the appropriateness of shocks was assessed. Patients with untreated lead malfunction or excessive inappropriate shocks were excluded from the study. Patients were divided into three groups: the first group included patients who had received no treatment, the second included patients with arrhythmia who received appropriate therapies but did not meet the criteria of arrhythmia storm, and the third included patients who had sustained VT or VF requiring three or more separate ICD therapies within 24 hours.
Statistical analysisAll the data entered into the database were verified by a second person. The variables were investigated to determine when they are normally distributed. Numerical variables were expressed as mean ± standard deviation for normally distributed variables and median (minimum-maximum) for skew-distributed continuous variables. Categorical variables were expressed as frequencies. Two groups were compared with the independent sample t test or the Mann-Whitney U test as appropriate. The chi-square test with Yates correction and Fisher's exact test were used for 2×2 contingency tables as appropriate for non-numerical data. Correlations between numerical parameters were analyzed with Pearson's or Spearman's rho correlation tests when required. A p-value <0.05 was accepted as significant. Receiver operating curve (ROC) analysis was performed. SPSS software was used for the statistical analysis.
ResultsStudy populationA total of 105 adults with ICDs treated in the cardiac arrhythmia outpatient clinic of a university hospital between January and April 2017 were analyzed. Of these, 19 patients who received DDD-ICDs or cardiac resynchronization therapy devices were excluded. Of the 86 patients with VVI-ICDs, 29 with a VVI-ICD for non-ischemic dilated cardiomyopathy, ARVD, Brugada syndrome, long or short QT syndrome, or hypertrophic cardiomyopathy were excluded from the study. Among the patients with a VVI-ICD and a diagnosis of ischemic dilated cardiomyopathy, three with lead defects, eight with inappropriate shocks, four with renal insufficiency, and 10 who did not consent to participation in the study, were excluded from the analysis.
Baseline characteristics of the study populationA total of 32 outpatients and 19 healthy controls participated in the study. Of the 32 patients, 87.5% (n=28) were men and 12.5% (n=4) were women. Mean age was 66.6±9.1 years. Of the control group, 63% (n=12) were men, 37% (n=7) were women, and mean age was 51.9±8.6 years. Demographic and clinical data of the patients are shown in Table 1. The characterization of tachyarrhythmia events obtained from VVI-ICD device analysis in the patient groups is shown in Table 2.
Demographic and clinical characteristics of the patient population.
| Gender | |
| Men, n (%) | 28 (87.5%) |
| Women, n (%) | 4 (12.5) |
| Age, years | 66.6±9.1 |
| MI location | |
| Anterior, n (%) | 9 (28.1%) |
| Inferior, n (%) | 7 (21.9%) |
| CABG, n (%) | 14 (43.8%) |
| EF, % | 36±7.7 |
| Diabetes, n (%) | 9 (28.1%) |
| Hypertension, n (%) | 13 (40.6%) |
| COPD, n (%) | 4 (12.5%) |
| Smoking, n (%) | 15 (46.9%) |
| Medication | |
| ACEi/ARB, n (%) | 28 (87.5%) |
| Beta-blocker, n (%) | 29 (90.6%) |
| Statin, n (%) | 29 (90.6%) |
| Spironolactone, n (%) | 15 (46.9%) |
| Amiodarone, n (%) | 15 (46.9%) |
| Sotalol, n (%) | 1 (3.1%) |
| Propafenone, n (%) | 1 (3.1%) |
ACEi/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; EF: ejection fraction; MI: myocardial infarction.
Characterization of tachyarrhythmic events obtained from VVI-ICD device analysis.
| Patient no. | Group | EF, % | No. of events | Type of event | Shock/ATP | Time since last event |
|---|---|---|---|---|---|---|
| 1 | 1 | 40 | 3 | Non-sustained VT | - | 30 month |
| 2 | 1 | 48 | 6 | Sustained VT- | - | 28 month |
| 3 | 1 | 27 | 7 | Non-sustained VT | - | 12 month |
| 4 | 1 | 32 | 3 | Sustained VT | - | 3 month |
| 5 | 1 | 31 | 1 | Sustained VT | - | 35 month |
| 6 | 1 | 27 | 5 | Non-sustained VT | - | 24 month |
| 7 | 1 | 56 | 1 | Sustained VT | - | 20 month |
| 8 | 1 | 46 | 0 | - | - | - |
| 9 | 1 | 34 | 10 | Non-sustained VT | - | 10 month |
| 10 | 1 | 37 | 0 | - | - | - |
| 11 | 1 | 39 | 1 | Sustained VT | - | 16 month |
| 12 | 1 | 34 | 1 | Sustained VT | - | 21 month |
| 13 | 1 | 35 | 0 | - | - | - |
| 14 | 1 | 40 | 0 | - | - | - |
| 15 | 1 | 35 | 17 | Non-sustained VT | - | 8 month |
| 16 | 1 | 33 | 1 | Sustained VT | - | 36 month |
| 17 | 2 | 40 | 2 | Sustained VT | 2 ATP/1 shock | 27 month |
| 18 | 2 | 50 | 15 | Sustained VT, VF | 12 ATP/3 shocks | 11 month |
| 19 | 2 | 36 | 2 | Sustained VT | 2 ATP | 31 month |
| 20 | 2 | 30 | 7 | Sustained VT, VF | 5 ATP/4 shocks | 2 month |
| 21 | 2 | 39 | 11 | Sustained VT, non-sustained VT | 7 ATP | 22 month |
| 22 | 2 | 42 | 7 | Sustained VT | 4 ATP/1 shock | 15 month |
| 23 | 3 | 25 | 4 | Sustained VT, arrhythmia storma | 11 ATP/7 shocks | 6 month |
| 24 | 3 | 30 | 2 | Arrhythmia storm | 11 ATP/9 shocks | 30 month |
| 25 | 3 | 35 | 34 | Sustained VT, VF, arrhythmia storm | 57 ATP/48 shocks | 13 month |
| 26 | 3 | 41 | 90 | Sustained VT, VF, arrhythmia storm | 70 ATP/130 shocks | 5 month |
| 27 | 3 | 24 | 11 | Sustained VT, VF, arrhythmia storm | 6 ATP/17 shocks | 12 month |
| 28 | 3 | 34 | 3 | Sustained VT, VF, arrhythmia storm | 4 ATP/7 shocks | 32 month |
| 29 | 3 | 36 | 3 | Arrhythmia storm | 11 ATP/8 shocks | 29 month |
| 30 | 3 | 46 | 7 | Sustained VT, VF, arrhythmia storm | 11 ATP/18 shocks | 21 month |
| 31 | 3 | 36 | 9 | Sustained VT, VF, arrhythmia storm | 12 ATP/17 shocks | 17 month |
| 32 | 3 | 21 | 38 | Sustained VT, arrhythmia storm | 29 ATP/12 shocks | 4 month |
The first group consisted of 16 patients, the second of six patients, and the third of 10 patients.
Comparison of patients in each subgroup revealed that a higher percentage of patients had diabetes in group three (p<0.05). However, there were no differences in terms of age, gender, ejection fraction (EF), glomerular filtration rate, hypertension, chronic obstructive lung disease or peripheral arterial disease.
Ventricular arrhythmias requiring therapies were identified in seven of the diabetic patients (87.5%), while this rate was significantly lower in non-diabetic patients (37.5%, p=0.03). Diabetic patients had significantly higher Gal-3 levels than non-diabetic patients (28.73±26.92 vs. 12.54±7.41 ng/ml, p=0.02).
Hypertensive patients had a similar risk of arrhythmia requiring ICD therapies to those without hypertension (46.6% vs. 52.6%, p>0.05).
The age of patients with arrhythmias requiring ICD therapies was similar to that of patients without arrhythmias (64.56±7.30 years vs. 68.56±10.36 years, p>0.05).
The difference between the mean EF of those with and those without MI was not significant (37±7.12% vs. 35.43±8.6, p>0.05).
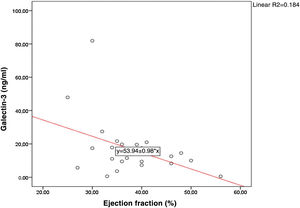
Mean Gal-3 values of those with MI were similar to those in non-MI patients (17.42±20.67 vs. 16.71±11.56 ng/ml, p>0.05). There was a significant negative correlation between the EF values of the patient group and Gal-3 levels (r=-0.429, p=0.03) (Figure 1).
Comparison of serum galectin-3 levels in patients and in controlsGal-3 levels were significantly higher in the patient groups than in controls (15.9±16.74 vs. 6.04±3.88 ng/ml, p<0.01).
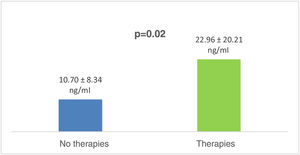
Comparison of serum galectin-3 levels between the three patient subgroupsGal-3 levels of patients with arrhythmias requiring ICD therapies were significantly higher than in patients with ICD not requiring therapies (22.96±20.21 vs. 10.70±8.34 ng/ml, p=0.02) (Figure 2).
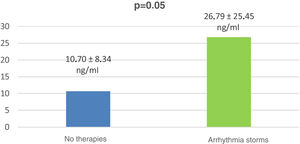
Gal-3 levels of patients with arrhythmia storms were significantly higher than in patients with no ICD therapies (26.79±25.45 vs. 10.70±8.34 ng/ml, p=0.05) (Figure 3).
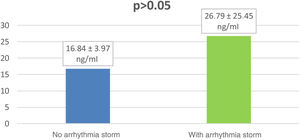
Gal-3 levels in patients requiring ICD therapies with arrhythmia storm were higher (but not significantly) than in patients requiring therapies without arrhythmia storm (26.79±25.45 vs. 16.84±3.97 ng/ml, p=0.94) (Figure 4).
Comparison of patients who received an implantable cardioverter-defibrillator for primary prevention and secondary preventionFourteen of the 32 patients received an ICD for primary prevention and 18 for secondary prevention.
The rate of prior arrhythmic events requiring ICD therapies was significantly higher in patients with an ICD for secondary prevention than in patients with an ICD for primary prevention (66.6% vs. 28.5%, p=0.03).
The mean EF of patients who received an ICD for primary prevention was similar to that of patients who received an ICD for secondary prevention (35.28%±5.13 vs. 36.94%±9.36, p>0.05).
Mean Gal-3 levels of patients who received an ICD for primary prevention purposes were similar to those of patients who received an ICD for secondary prevention (14.16±7.27 vs. 19.02±20.68 ng/ml, p>0.05).
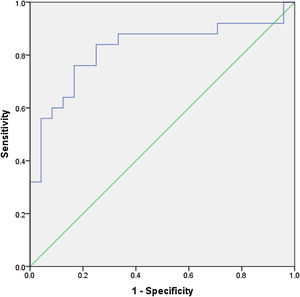
Receiver operating characteristic curve analysis for galectin-3ROC analysis showed with 84% sensitivity and 75% specificity that Gal-3 levels over 7 ng/ml identified ventricular arrhythmias that required therapies (area under the curve: 0.823 [95% confidence interval 0.698-0.948]) (Figure 5).
DiscussionIn the present study we evaluated Gal-3 as a potential predictor of sustained VT/VF in ICD patients. Gal-3 levels were significantly higher in the patient groups than in healthy controls and in patients requiring ICD therapies than in those not requiring therapies, and were associated with the presence of arrhythmias that required ICD therapies. There was a significant negative correlation between EF and Gal-3 levels in the patient groups.
An ICD is recommended in patients with dilated cardiomyopathy, symptomatic heart failure (New York Heart Association class II or III) and EF ≤35% despite ≥3 months of optimal pharmacological therapy who are expected to survive for >1 year with good functional status. In a post-hoc analysis of the MADDIT II trial, only 35% of ICD patients received appropriate ICD therapies in a three-year follow-up.12 In another study, a significant proportion of patients resuscitated from sudden cardiac death had EF>35%.13 This suggests that some ICDs implanted in accordance with the current guidelines may be unnecessary, and that some patients who did not receive an ICD may have died from sudden death. Given the complications and financial burden of ICD use, additional parameters are needed to improve the indications for implantation.
Ventricular arrhythmias are thought to arise due to the heterogeneous structure of scar tissue and healthy peri-infarct tissue. This heterogeneous structure gives rise to slow and fast pathways that provide the basis for the reentry circuit and thus maintains the arrhythmia.14 Scar tissue in homogenous structures was shown to constitute less arrhythmia risk than in heterogeneous structures.15–19
The gold standard non-invasive imaging technique for detecting myocardial scar tissue is MRI. Wu et al. reported an eight-fold increase in hospitalization for heart failure, appropriate ICD firing and cardiac death in ischemic dilated cardiomyopathy patients with scar tissue as revealed by late gadolinium enhancement on cardiac MRI compared to those without scar tissue.8 This finding was supported by a study by Assomull et al. and in other studies.7,20 A study by Scott et al. showed that the magnitude of scar tissue, as demonstrated by late gadolinium enhancement on cardiac MRI in patients with coronary artery disease, increases the risk of ventricular arrhythmias independently of EF.17
In the damaged area, Gal-3 plays an important role in the fibrotic process by converting inactive fibroblasts to active myofibroblasts that produce collagen and glycoprotein.9,20,21 Gal-3 was shown to be secreted from myocardial scar tissue and to promote myocardial fibrosis and cardiac dysfunction. It has been associated with remodeling in patients with heart failure9 and Gal-3 levels secreted from fibrotic tissue have been identified as the best independent predictor of mortality in heart failure.10,22 Pietro et al. found that Gal-3 levels were a predictive marker for VT/VF in patients with heart failure who received an ICD, with sensitivity and specificity of 60% and 65%, respectively, for predicting VT/VF in patients with Gal-3>17 ng/ml.23
Some limitations should be considered when interpreting these data. The main limitation is the small number of patients. Secondly, cardiac structure and arrhythmic burden were not assessed for the control group. Third, it is not possible to exclude non-cardiac causes that could increase plasma Gal-3 concentrations. Finally, arrhythmia is not solely caused by fibrosis. It may also occur due to ischemia and other electrophysiological causes.
ConclusionGal-3 may be an inexpensive and easily accessible parameter to predict arrhythmia risk in patients with ischemic dilated cardiomyopathy. Gal-3 may further improve risk stratification in ischemic cardiomyopathy patients who are more prone to developing life-threatening arrhythmias. However, large-scale prospective studies including efficacy and safety studies are needed to determine the role of this biomarker in predicting arrhythmias and sudden cardiac death in clinical practice.
FundingThe authors received no financial support for the research, authorship, or publication of this article.
Conflicts of interestThe authors have no conflicts of interest to declare.