The Fontan procedure and the management of patients with univentricular physiology have undergone significant evolution over the past five decades. However, the long-term outcomes of these patients remain not fully understood. This study aimed to evaluate the early and long-term outcomes of patients undergoing the Fontan procedure and to identify risk factors associated with adverse clinical events.
MethodsPatients who underwent the Fontan procedure between 2004 and 2023 were included in this study. Multivariable logistic regression analysis was employed to assess risk factors for early mortality, while a Cox proportional hazards regression model was used to evaluate predictors of long-term Fontan failure.
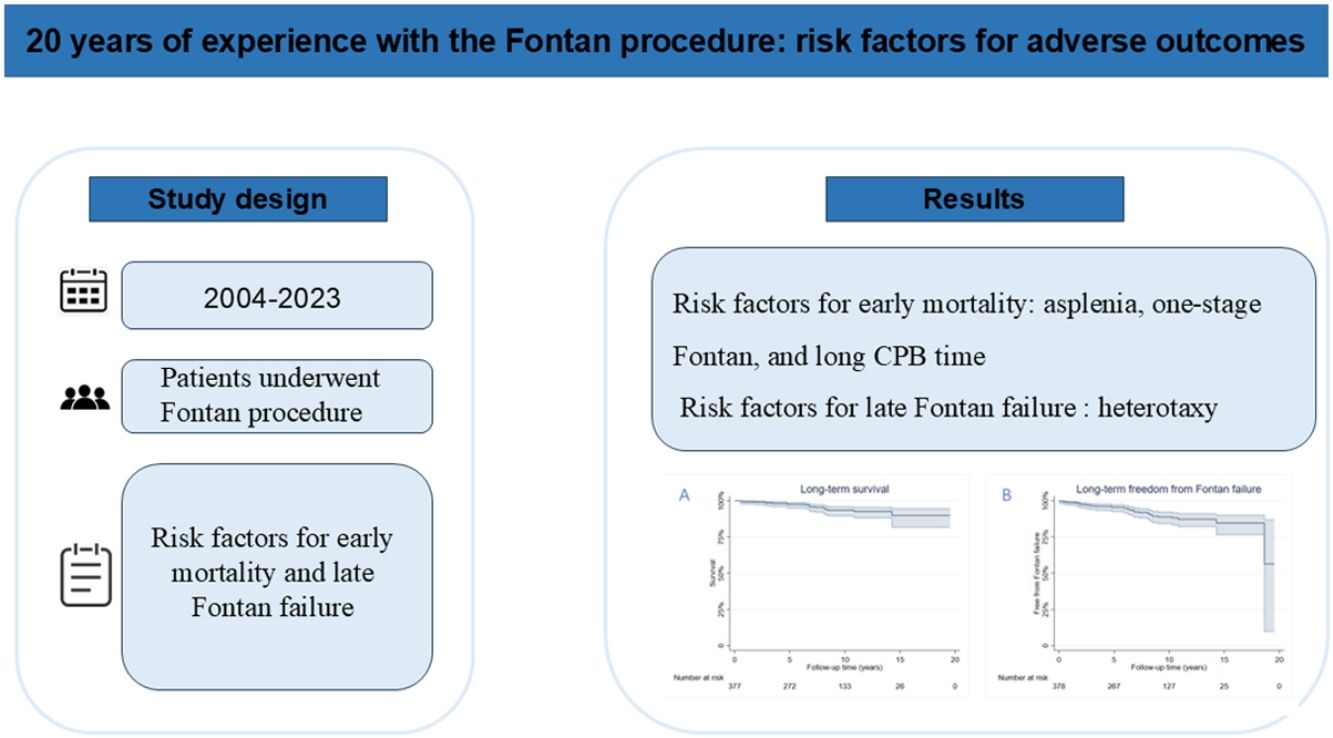
ResultsA total of 400 patients were included, with a male predominance (67.3%). Median age at the time of the Fontan procedure was 5.8 years (interquartile range: 4.1–11.0 years). The distribution of ventricular morphology was as follows: dominant right ventricle (33%), dominant left ventricle (35.75%), and two well-developed ventricles (28.75%). The early mortality rate was 5.5%. The overall survival rates at 5-, 10-, and 15-years post-Fontan surgery were 97.5%, 92.6%, and 90.0%, respectively. Multivariable analysis identified asplenia (odds ratio [OR], 10.2; 95% confidence interval [CI], 2.8–36.4; p<0.01), single-stage total cavopulmonary connection (OR, 5.3; 95% CI, 1.7–16.8; p<0.01), and prolonged cardiopulmonary bypass time (OR, 1.0; 95% CI, 1.0–1.0; p<0.01) as significant predictors of early mortality. Cox regression analysis demonstrated that heterotaxy (hazard ratio [HR], 3.5; 95% CI, 1.4–8.7; p<0.01) was an independent risk factor for late Fontan failure.
ConclusionThe staged Fontan strategy was associated with reduced early mortality but did not confer significant benefits on long-term outcomes. Patients with heterotaxy were at an increased risk of late Fontan failure, highlighting the need for tailored management strategies in this high-risk population.
O procedimento de Fontan e o tratamento de doentes com fisiologia univentricular evoluíram significativamente ao longo dos últimos 50 anos. No entanto, os resultados a longo prazo permanecem pouco compreendidos. Este estudo teve como objetivo avaliar os resultados precoces e a longo prazo de doentes submetidos ao procedimento de Fontan e identificar fatores de risco associados a eventos clínicos adversos.
MétodosDoentes submetidos ao procedimento de Fontan entre 2004 e 2023 foram incluídos neste estudo. A análise de regressão logística multivariável foi utilizada para avaliar fatores de risco para mortalidade precoce, enquanto um modelo de regressão de Cox foi empregue para avaliar preditores de insuficiência de Fontan a longo prazo.
ResultadosUm total de 400 doentes foi incluído, com predomínio do sexo masculino (67,3%). A mediana de idade no momento do procedimento de Fontan foi de 5,8 anos (intervalo interquartil [IIQ]: 4,1–11,0 anos). A distribuição da morfologia ventricular foi a seguinte: ventrículo direito dominante (33%), ventrículo esquerdo dominante (35,75%) e dois ventrículos bem desenvolvidos (28,75%). A taxa de mortalidade precoce foi de 5,5%. As taxas de sobrevida global aos 5, 10 e 15 anos após a cirurgia de Fontan foram de 97,5%, 92,6% e 90,0%, respetivamente. A análise multivariável identificou asplenia (razão de chances [OR], 10,2; intervalo de confiança [IC] de 95%, 2,8–36,4; p < 0,01), conexão cavopulmonar total em estágio único (OR, 5,3; IC 95%, 1,7–16,8; p < 0,01) e tempo prolongado de circulação extracorpórea (OR, 1,0; IC 95%, 1,0–1,0; p < 0,01) como preditores significativos de mortalidade precoce. A análise de regressão de Cox demonstrou que a heterotaxia (razão de risco [HR], 3,5; IC 95%, 1,4–8,7; p < 0,01) foi um fator de risco independente para insuficiência de Fontan a longo prazo.
ConclusãoA estratégia de Fontan em estágios foi associada à redução da mortalidade precoce, mas não conferiu benefícios significativos aos resultados a longo prazo. Os doentes com heterotaxia apresentaram maior risco de insuficiência de Fontan a longo prazo, destacando a necessidade de estratégias de tratamento personalizadas para essa população de alto risco.
Fontan and Baudet1 initially described the Fontan operation, which involved applying right ventricular exclusion to a patient with tricuspid atresia. The results of patients with univentricular physiology have improved over the years due to a variety of successive advancements in surgical procedures.2–4 Total cavopulmonary connection (TCPC) as a contemporary approach was established. However, despite the improvements in outcomes, Fontan patients still face significant challenges.5–7 Although patients can have a good function status with a well-functioning Fontan circulation, numerous complications will occur, which affect their life expectancy.8–10 The complications include heart failure, protein-losing enteropathy (PLE), plastic bronchitis (PB), Fontan-associated liver disease (FALD), arrhythmias, and thrombosis. Patients who suffer from Fontan failure will pose challenges to management.11 Therefore, the long-term outcome of the Fontan procedure is still being understood.
ObjectivesThe aim of our study was to estimate the early and long-term outcomes of Fontan patients and to investigate the risk factors for adverse outcomes.
MethodsStudy populationThis study was approved by the Institutional Review Board at our hospital (GDREC2019338H) with a waiver for consent as it was a retrospective study. Patients who underwent Fontan procedure from 2004 to 2023 were included. Those with insufficient data were excluded. The demographic, perioperative, and hemodynamic data were collected from review of the electronic medical record. The follow-up data was obtained from outpatient records and by contacting patients. The primary outcomes of interest were early mortality and late Fontan failure.
DefinitionsSignificant common atrioventricular valve regurgitation (AVVR) was defined as moderate or severe AVVR. Early mortality was defined as death occurring within the first 30 days after Fontan procedure or before discharge. Fontan failure was defined as death, takedown, transplantation, or PLE.
Statistical analysisContinuous variables were tested with the Kolmogorov–Smirnov test for normal distribution, which are presented as mean±standard deviation for normal or median (interquartile range [IQR]) for non-normal distribution. Categorical data were summarized as frequencies and percentages. Survival and the incidence of late Fontan failure curves were obtained using the Kaplan–Meier method. Multivariable logistic regression analysis was used to identify factors relating to early mortality. A Cox-proportion regression hazard model was used to estimate the risk of long-term Fontan failure.
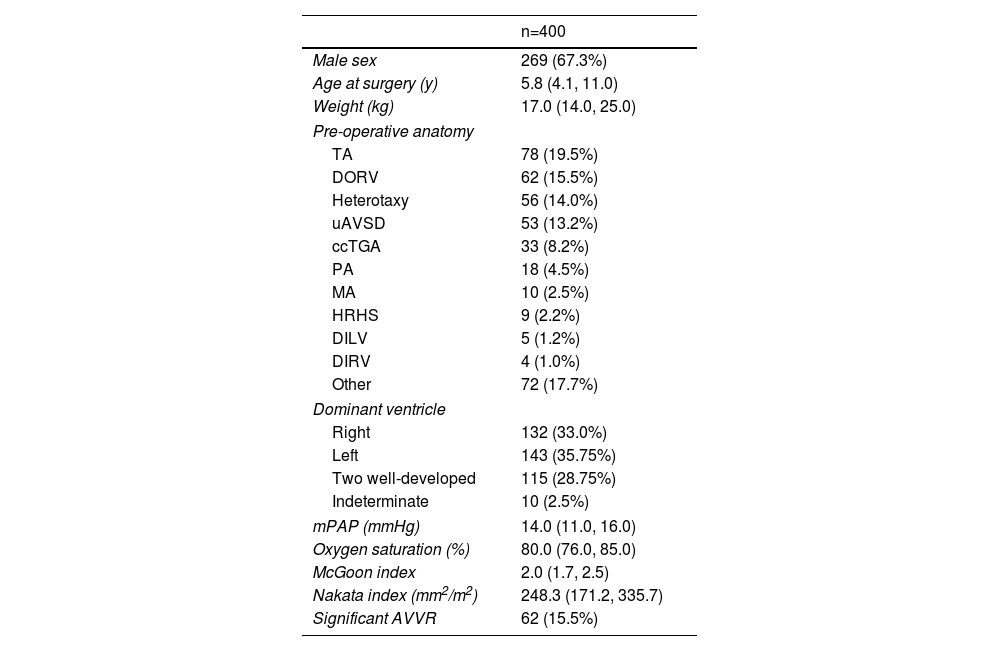
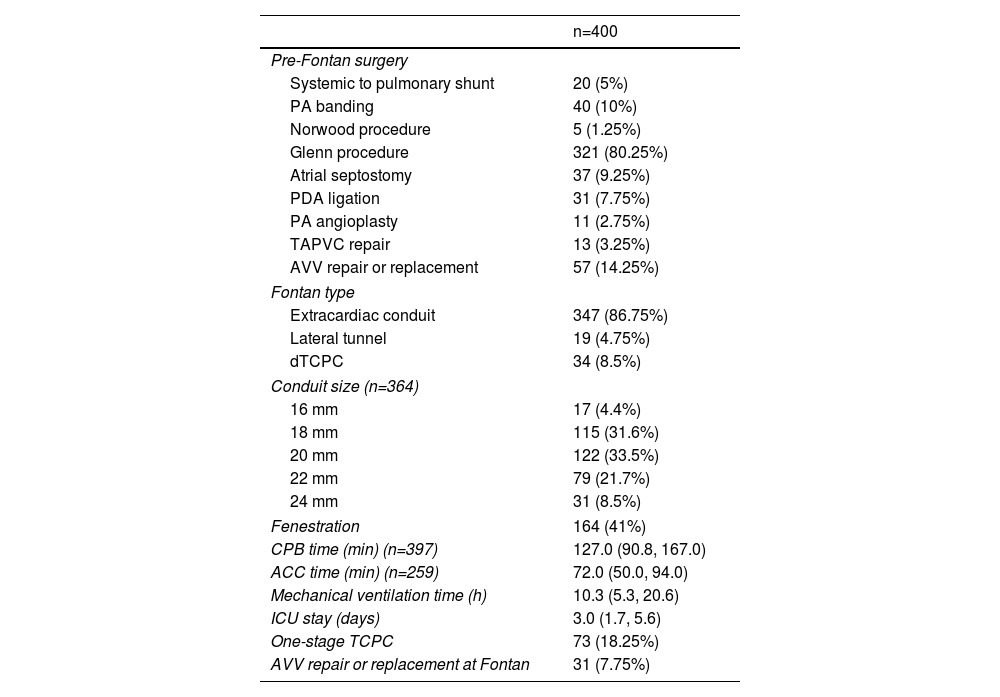
ResultsBaseline characteristicsThe study cohort comprised 400 consecutive patients who underwent Fontan procedures at our institution. Baseline demographic and clinical characteristics are detailed in Table 1. The median age at Fontan completion was 5.8 years (IQR: 4.1–11.0 years), with male predominance (67.3%, n=269). The most common preoperative anatomy included tricuspid atresia (TA) (19.5%, n=80), double-outlet right ventricle (DORV) (15.5%, n=62), heterotaxy syndrome (14.0%, n=56), and unbalanced atrioventricular septal defect (uAVSD) (13.2%, n=53). Ventricular morphology analysis revealed right ventricular dominance in 33.0% (n=132), left ventricular dominance in 35.8% (n=143), and biventricular morphology in 28.8% (n=115) of cases. Preoperative significant atrioventricular valve regurgitation (AVVR) was present in 15.5% (n=62) of patients.
Baseline characteristics.
| n=400 | |
|---|---|
| Male sex | 269 (67.3%) |
| Age at surgery (y) | 5.8 (4.1, 11.0) |
| Weight (kg) | 17.0 (14.0, 25.0) |
| Pre-operative anatomy | |
| TA | 78 (19.5%) |
| DORV | 62 (15.5%) |
| Heterotaxy | 56 (14.0%) |
| uAVSD | 53 (13.2%) |
| ccTGA | 33 (8.2%) |
| PA | 18 (4.5%) |
| MA | 10 (2.5%) |
| HRHS | 9 (2.2%) |
| DILV | 5 (1.2%) |
| DIRV | 4 (1.0%) |
| Other | 72 (17.7%) |
| Dominant ventricle | |
| Right | 132 (33.0%) |
| Left | 143 (35.75%) |
| Two well-developed | 115 (28.75%) |
| Indeterminate | 10 (2.5%) |
| mPAP (mmHg) | 14.0 (11.0, 16.0) |
| Oxygen saturation (%) | 80.0 (76.0, 85.0) |
| McGoon index | 2.0 (1.7, 2.5) |
| Nakata index (mm2/m2) | 248.3 (171.2, 335.7) |
| Significant AVVR | 62 (15.5%) |
AVVR: atrioventricular valve regurgitation; ccTGA: congenitally corrected transposition of the great arteries; DILV: double-inlet left ventricle; DIRV: double-inlet right ventricle; DORV: double-outlet right ventricle; HRHS: hypoplastic right heart syndrome; MA: mitral atresia; mPAP: mean pulmonary artery pressure; PA: pulmonary atresia; TA: tricuspid atresia; TAPVC: total anomalous pulmonary venous connection; TGA: transposition of the great arteries; uAVSD: unbalanced atrioventricular septal defect.
Perioperative data are presented in Table 2. The extracardiac conduit TCPC technique was employed in 86.8% (n=347) of cases, with conduit sizes of 18 mm (31.6%, n=126) and 20 mm (33.5%, n=134) being most frequently utilized. Surgical approaches included off-pump TCPC in 0.8% (n=3) and on-pump beating TCPC in 35.3% (n=141) of patients. The median cardiopulmonary bypass (CPB) time was 127.0 minutes (IQR: 90.8–167.0 minutes). One-stage TCPC was performed in 18.3% (n=73) of cases. Concomitant atrioventricular valve procedures (valvuloplasty or replacement) were required in 7.8% (n=31) of patients due to significant AVVR. Pre-Fontan palliative procedures included systemic-to-pulmonary shunts (5.0%, n=20), pulmonary artery banding (10.0%, n=40), Norwood procedure (1.3%, n=5), and bidirectional Glenn shunt (80.3%, n=321).
Perioperative characteristics.
| n=400 | |
|---|---|
| Pre-Fontan surgery | |
| Systemic to pulmonary shunt | 20 (5%) |
| PA banding | 40 (10%) |
| Norwood procedure | 5 (1.25%) |
| Glenn procedure | 321 (80.25%) |
| Atrial septostomy | 37 (9.25%) |
| PDA ligation | 31 (7.75%) |
| PA angioplasty | 11 (2.75%) |
| TAPVC repair | 13 (3.25%) |
| AVV repair or replacement | 57 (14.25%) |
| Fontan type | |
| Extracardiac conduit | 347 (86.75%) |
| Lateral tunnel | 19 (4.75%) |
| dTCPC | 34 (8.5%) |
| Conduit size (n=364) | |
| 16 mm | 17 (4.4%) |
| 18 mm | 115 (31.6%) |
| 20 mm | 122 (33.5%) |
| 22 mm | 79 (21.7%) |
| 24 mm | 31 (8.5%) |
| Fenestration | 164 (41%) |
| CPB time (min) (n=397) | 127.0 (90.8, 167.0) |
| ACC time (min) (n=259) | 72.0 (50.0, 94.0) |
| Mechanical ventilation time (h) | 10.3 (5.3, 20.6) |
| ICU stay (days) | 3.0 (1.7, 5.6) |
| One-stage TCPC | 73 (18.25%) |
| AVV repair or replacement at Fontan | 31 (7.75%) |
ACC: aortic cross clamping; AVV: atrioventricular valve; CPB: cardiopulmonary bypass; dTCPC: direct total cavopulmonary connection; ICU: intensive care unit; PA: pulmonary artery; PDA: patent ductus arteriosus; TAPVC: total anomalous pulmonary venous connection; TCPC: total cavopulmonary connection.
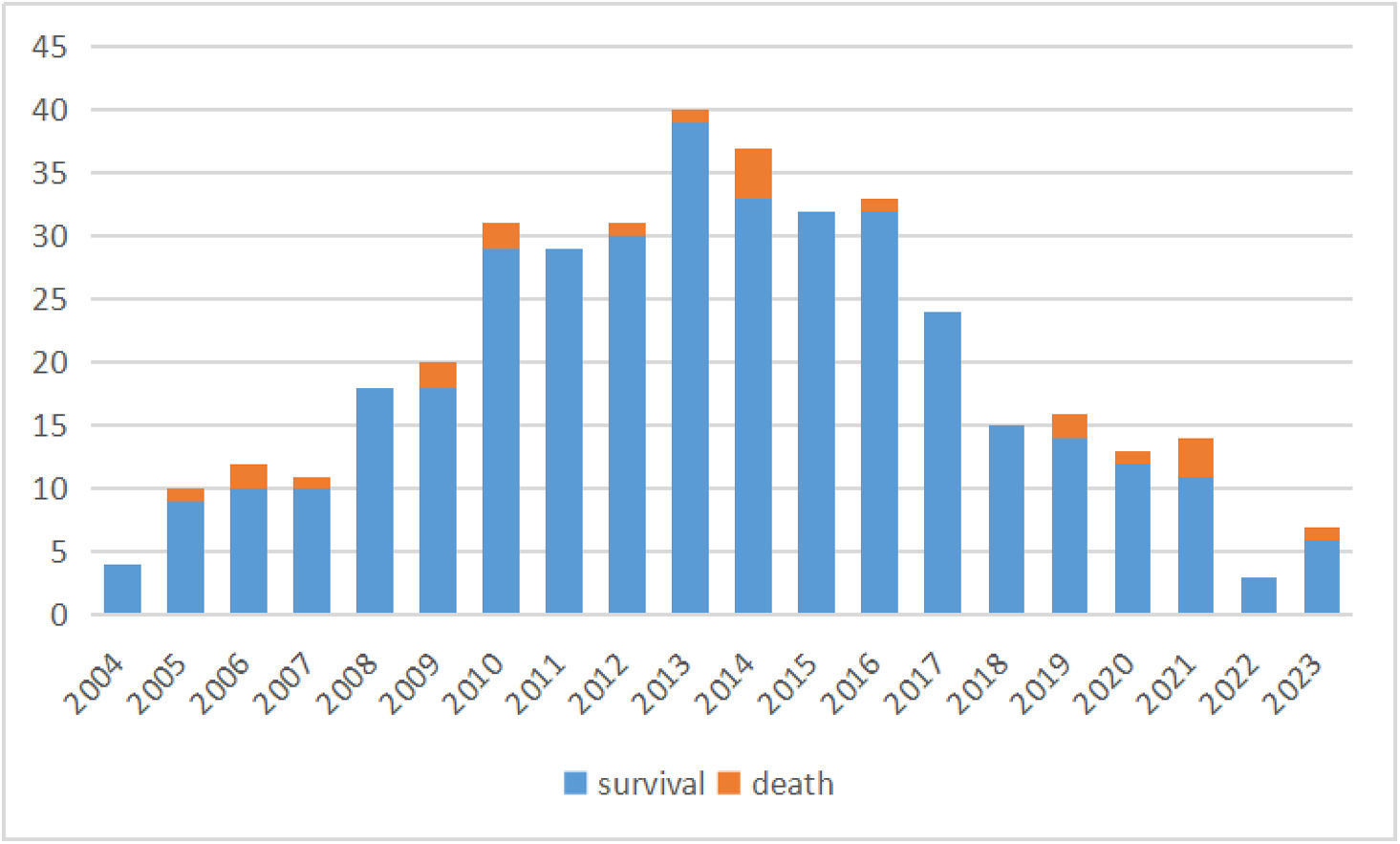
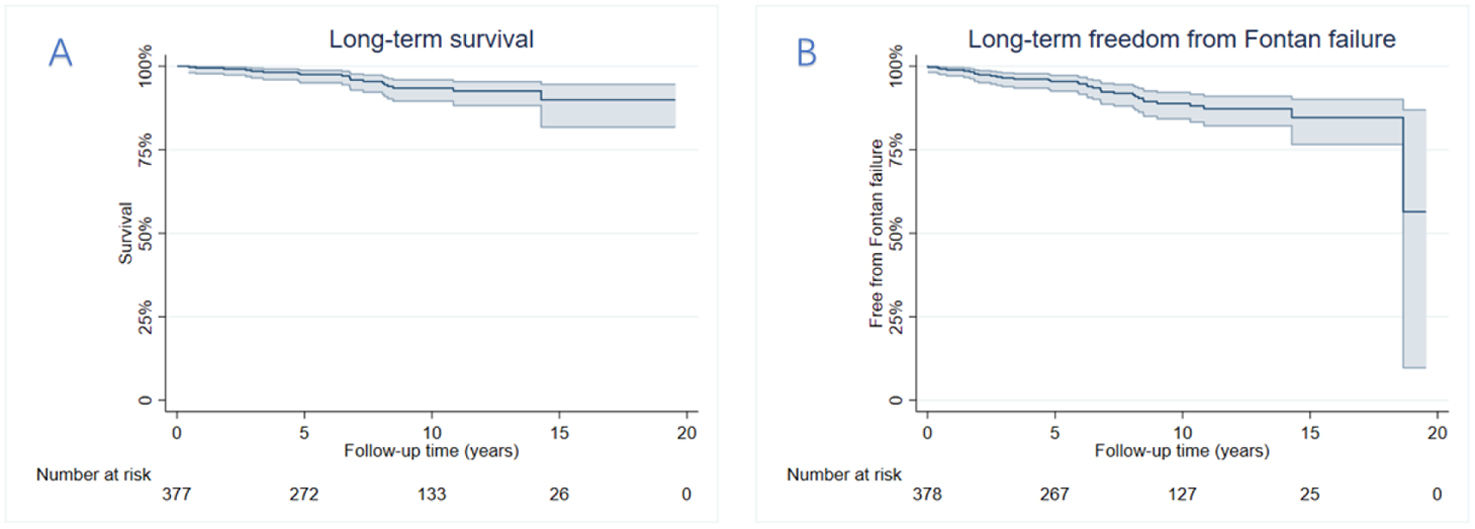
Early postoperative mortality occurred in 5.5% (n=22) of cases, with primary causes including low cardiac output syndrome, sepsis, multiple organ dysfunction syndrome, disseminated intravascular coagulation, and sudden cardiac death. The number of operations and deaths in each year has been summarized in Figure 1. The median follow-up duration was 8.4 years (IQR: 3.8–12.3 years). Late Fontan failure was observed in 8.5% (n=34) of patients, with major complications including arrhythmias (16.0%, n=64), PLE (5.0%, n=20), and thromboembolic events (2.3%, n=9). Late mortality occurred in 4.8% (n=19) of cases, predominantly due to heart failure, cerebrovascular events, coagulation disorders, respiratory failure, malignant arrhythmias, and sudden cardiac death. Cardiac transplantation was required in 0.5% (n=2) of patients following circulatory failure. Kaplan–Meier analysis demonstrated overall survival rates of 97.5%, 92.6%, and 90.0% at 5, 10, and 15 years postoperatively, respectively (Figure 2). Free from Fontan failure rates were 95.5%, 88.1%, and 84.6% at the corresponding time points (Table 3).
Kaplan–Meier curve of (A) the overall survival curve and (B) freedom from late Fontan failure. The overall survival rates after Fontan surgery were 97.5%, 92.6%, 90.0% at 5, 10, 15 years, respectively. Overall freedom from Fontan failure was 95.5%, 88.1%, and 84.6% at 5, 10, and 15 years, respectively.
Early and long-term outcomes.
| Early outcome | n=400 |
|---|---|
| Early death | 22 (5.5%) |
| Takedown | 6 (1.5%) |
| Prolonged hospital stays | 101 (25.25%) |
| Long-term outcome | n=378 |
|---|---|
| Arrhythmia | 64 (16.9%) |
| Late Fontan failure | 34 (9.0%) |
| Late death | 19 (5.0%) |
| Protein-losing enteropathy | 20 (5.3%) |
| Fontan-associated liver disease | 13 (3.4%) |
| Thromboembolic events | 9 (2.3%) |
| Transplantation | 2 (0.5%) |
| Takedown | 1 (0.3%) |
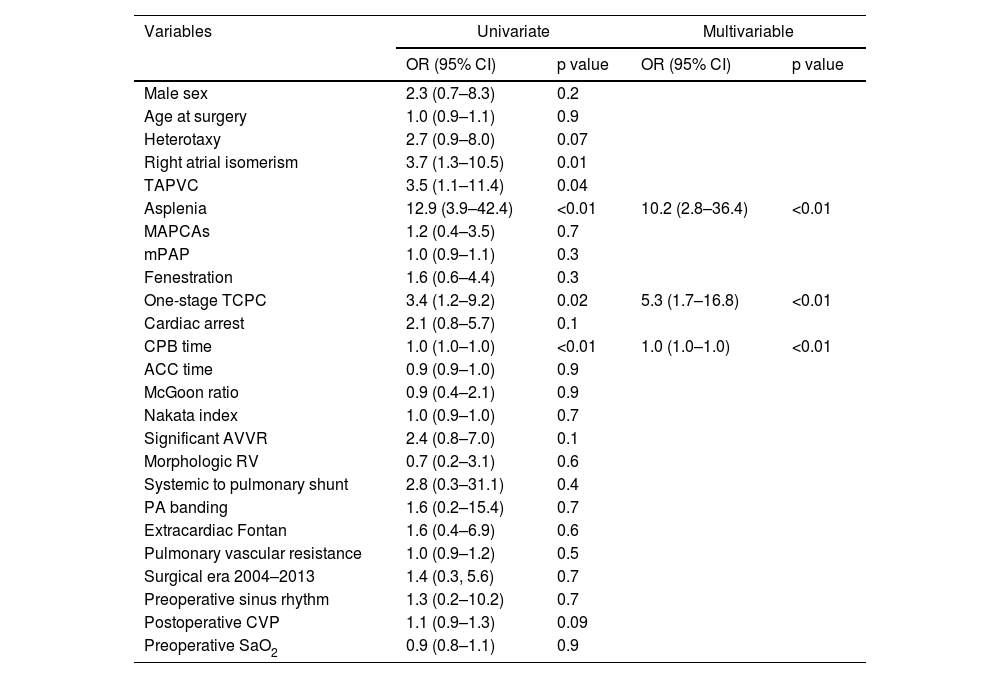
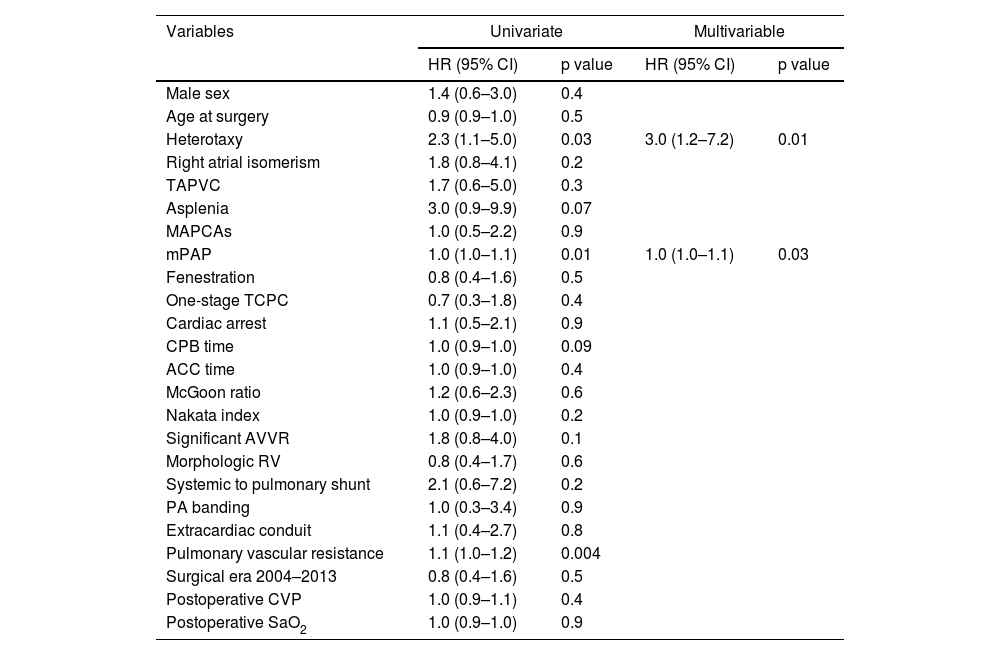
Multivariable logistic regression identified asplenia as the most significant predictor of early mortality (odds ratio [OR]: 10.2; 95% confidence interval [CI]: 2.8–36.4; p<0.01), conferring a 10-fold increased risk of death within 30 days postoperatively or before hospital discharge (Table 4). Additional independent risk factors for early mortality included single-stage TCPC (OR: 5.3; 95% CI: 1.7–16.8; p<0.01) and prolonged CPB time (OR: 1.0 per minute increase; 95% CI: 1.0–1.0; p<0.01). Cox proportional hazards analysis revealed heterotaxy syndrome (hazard ratio [HR]: 3.0; 95% CI: 1.2–7.2; p=0.01) and mPAP (HR: 1.0; 95% CI: 1.0–1.1) as significant predictors of late Fontan failure (Table 5).
Univariable and multivariable logistic regression analysis for risk factors of early mortality.
| Variables | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Male sex | 2.3 (0.7–8.3) | 0.2 | ||
| Age at surgery | 1.0 (0.9–1.1) | 0.9 | ||
| Heterotaxy | 2.7 (0.9–8.0) | 0.07 | ||
| Right atrial isomerism | 3.7 (1.3–10.5) | 0.01 | ||
| TAPVC | 3.5 (1.1–11.4) | 0.04 | ||
| Asplenia | 12.9 (3.9–42.4) | <0.01 | 10.2 (2.8–36.4) | <0.01 |
| MAPCAs | 1.2 (0.4–3.5) | 0.7 | ||
| mPAP | 1.0 (0.9–1.1) | 0.3 | ||
| Fenestration | 1.6 (0.6–4.4) | 0.3 | ||
| One-stage TCPC | 3.4 (1.2–9.2) | 0.02 | 5.3 (1.7–16.8) | <0.01 |
| Cardiac arrest | 2.1 (0.8–5.7) | 0.1 | ||
| CPB time | 1.0 (1.0–1.0) | <0.01 | 1.0 (1.0–1.0) | <0.01 |
| ACC time | 0.9 (0.9–1.0) | 0.9 | ||
| McGoon ratio | 0.9 (0.4–2.1) | 0.9 | ||
| Nakata index | 1.0 (0.9–1.0) | 0.7 | ||
| Significant AVVR | 2.4 (0.8–7.0) | 0.1 | ||
| Morphologic RV | 0.7 (0.2–3.1) | 0.6 | ||
| Systemic to pulmonary shunt | 2.8 (0.3–31.1) | 0.4 | ||
| PA banding | 1.6 (0.2–15.4) | 0.7 | ||
| Extracardiac Fontan | 1.6 (0.4–6.9) | 0.6 | ||
| Pulmonary vascular resistance | 1.0 (0.9–1.2) | 0.5 | ||
| Surgical era 2004–2013 | 1.4 (0.3, 5.6) | 0.7 | ||
| Preoperative sinus rhythm | 1.3 (0.2–10.2) | 0.7 | ||
| Postoperative CVP | 1.1 (0.9–1.3) | 0.09 | ||
| Preoperative SaO2 | 0.9 (0.8–1.1) | 0.9 | ||
ACC: aortic cross clamp; AVVR: atrioventricular valve regurgitation; CI: confidence interval; CPB: cardiopulmonary bypass; CVP: central venous pressure; MAPCAs: major aortopulmonary collateral arteries; mPAP: mean pulmonary artery pressure; OR: odds ratio; PA: pulmonary artery; SaO2: oxygen saturation; TAPVC: total anomalous pulmonary venous connection; TCPC: total cavopulmonary connection.
Cox regression model for risk factors of late Fontan failure.
| Variables | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Male sex | 1.4 (0.6–3.0) | 0.4 | ||
| Age at surgery | 0.9 (0.9–1.0) | 0.5 | ||
| Heterotaxy | 2.3 (1.1–5.0) | 0.03 | 3.0 (1.2–7.2) | 0.01 |
| Right atrial isomerism | 1.8 (0.8–4.1) | 0.2 | ||
| TAPVC | 1.7 (0.6–5.0) | 0.3 | ||
| Asplenia | 3.0 (0.9–9.9) | 0.07 | ||
| MAPCAs | 1.0 (0.5–2.2) | 0.9 | ||
| mPAP | 1.0 (1.0–1.1) | 0.01 | 1.0 (1.0–1.1) | 0.03 |
| Fenestration | 0.8 (0.4–1.6) | 0.5 | ||
| One-stage TCPC | 0.7 (0.3–1.8) | 0.4 | ||
| Cardiac arrest | 1.1 (0.5–2.1) | 0.9 | ||
| CPB time | 1.0 (0.9–1.0) | 0.09 | ||
| ACC time | 1.0 (0.9–1.0) | 0.4 | ||
| McGoon ratio | 1.2 (0.6–2.3) | 0.6 | ||
| Nakata index | 1.0 (0.9–1.0) | 0.2 | ||
| Significant AVVR | 1.8 (0.8–4.0) | 0.1 | ||
| Morphologic RV | 0.8 (0.4–1.7) | 0.6 | ||
| Systemic to pulmonary shunt | 2.1 (0.6–7.2) | 0.2 | ||
| PA banding | 1.0 (0.3–3.4) | 0.9 | ||
| Extracardiac conduit | 1.1 (0.4–2.7) | 0.8 | ||
| Pulmonary vascular resistance | 1.1 (1.0–1.2) | 0.004 | ||
| Surgical era 2004–2013 | 0.8 (0.4–1.6) | 0.5 | ||
| Postoperative CVP | 1.0 (0.9–1.1) | 0.4 | ||
| Postoperative SaO2 | 1.0 (0.9–1.0) | 0.9 | ||
ACC: aortic cross clamp; AVVR: atrioventricular valve regurgitation; CI: confidence interval; CPB: cardiopulmonary bypass; CVP: central venous pressure; MAPCAs: major aortopulmonary collateral arteries; mPAP: mean pulmonary artery pressure; OR: odds ratio; PA: pulmonary artery; SaO2: oxygen saturation; TAPVC: total anomalous pulmonary venous connection; TCPC: total cavopulmonary connection.
In this study, we evaluated the clinical outcomes of patients undergoing the Fontan procedure. The primary findings were as follows: (1) The early mortality rate was 5.5%, with significant associations observed with asplenia, one-stage TCPC, and prolonged CPB time. (2) The incidence of Fontan failure was 9.0%, which was significantly influenced by the presence of heterotaxy and elevated pulmonary artery pressure.
Most of our patients underwent extracardiac conduit TCPC, whereas the lateral tunnel technique was less frequently utilized. Although computational fluid dynamics simulation models have not demonstrated a clear advantage of the extracardiac conduit over the lateral tunnel,12 the extracardiac approach offers the benefit of avoiding aortic cross clamping and intra-atrial suturing. In our cohort, 35.4% of procedures were performed without cross clamping, which theoretically may reduce the incidence of arrhythmias. A recent meta-analysis by Ben Ali et al.13 supports this notion, indicating that the extracardiac conduit Fontan procedure preserves sinus node function and significantly reduces the incidence of long-term postoperative arrhythmias. Furthermore, studies have demonstrated more favorable long-term survival and exercise outcomes with the extracardiac conduit.14,15 However, the adequacy of conduit size remains a critical concern due to its lack of growth potential. Both oversized and undersized conduits are associated with significant morbidity and mortality. The modified Fontan procedure, utilizing a direct TCPC (dTCPC) with entirely autologous vessels, appears to offer a promising solution. As previously reported,16 we have investigated this novel approach in selected patients, yielding favorable long-term outcomes and potential for growth.
Following multiple modifications to the Fontan procedure, both early and late postoperative mortality rates have significantly declined. In our cohort, the early mortality rate was 5.5%. One-stage TCPC was performed in 18.25% of cases but accounted for 40.9% of early deaths. The overall early mortality rate for one-stage TCPC was 12.3% (9/73), with most of these procedures (84.9%) performed within the first decade. Over time, staging of the Fontan palliation has been recognized as an optimal strategy to reduce mortality at Fontan completion, particularly in high-risk patients. Staged TCPC allows for gradual ventricular unloading, thereby improving hemodynamics. However, significant interstage mortality remains a concern,17 and the rate of final Fontan completion remains suboptimal even with a staged approach. Patients deemed unsuitable for eventual Fontan completion are often left with a bidirectional cavopulmonary shunt for extended periods. Post-Fontan completion, long-term survival rates were comparable between one-stage and staged TCPC.
Consistent with previous studies,18 we identified asplenia as a significant predictor of early mortality, strongly associated with heterotaxy in our cohort (14/17). Patients with complex anatomical variations, such as direct hepatic venous return to the atrium, often required additional surgical interventions. Furthermore, asplenia was associated with decreased long-term survival.19
With advancements in management strategies, the prospective survival of Fontan patients has steadily improved, surpassing earlier expectations. In our cohort, the overall survival rates post-Fontan surgery were 97.5%, 92.6%, and 90.0% at 5, 10, and 15 years, respectively. Despite these improvements, postoperative complications remain a significant challenge, necessitating lifelong management. Fontan failure represents the most common cause of death, with notable complications including PLE, PB, FALD, arrhythmias, significant AVVR, and thromboembolism.
The Fontan population continues to grow, driven by advancements in surgical techniques. However, Fontan circulation is not a definitive treatment and is associated with chronic multisystem morbidity. Risk stratification remains challenging, and sudden adverse events can occur unpredictably. A survey conducted by Elder et al.20 revealed that cardiologists were only marginally able to predict which Fontan patients were at risk for major adverse events over a one-year period. While risk factors for adverse events in Fontan patients have been extensively studied, consensus remains elusive. Late mortality and failure risk factors included significant AVVR, heterotaxy, high pulmonary arterial pressure, morphologic right ventricle. The risk factors for long-term outcomes vary in different studies. In our study, heterotaxy and elevated pulmonary artery pressure were associated with late Fontan failure. However, Fontan failure is difficult to predict with many patients seemingly clinically well with normal ventricular function. Significant differences in cardiac diagnoses further complicate risk stratification, underscoring the need for international collaboration to achieve robust risk assessment.
This study has several limitations that must be acknowledged. First, it was conducted at a single center, which may limit the generalizability of the findings. Second, the retrospective nature of the study introduces inherent biases related to patient selection and data availability.
ConclusionsThe early mortality rate was 5.5% while the overall survival rates after Fontan surgery were 97.5%, 92.6%, 90.0% at 5, 10, 15 years, respectively. Staging Fontan palliation reduced early mortality but did not show any benefit on long-term outcome. Asplenia and long CPB time were the other risk factors for early mortality. Heterotaxy was associated with late Fontan failure.
Ethical approvalThe current study was approved by the Institutional Review Board and Hospital Research Ethics Committee of Guangdong Provincial People's Hospital (GDREC2019338H).
FundingThis work was supported by the National Natural Science Foundation of China (grant number 82300268, 2024), Science and Technology Planning Project of Guangdong Province, China (No. 2019B020230003), Science and Technology Projects in Guangzhou, China (202206010049), Guangdong Special Funds for Science and Technology Innovation Strategy, China (Stability Support for Scientific Research Institutions Affiliated to Guangdong Province, No. KD022023019).
Conflicts of interestThe authors declare no conflict of interest.