Prosthetic valve endocarditis is a major diagnostic challenge in clinical practice, due to the lower sensitivity of the modified Duke criteria and a higher percentage of cases with negative or inconclusive echocardiography results. The delay in establishing medical and surgical treatment increases the morbidity/mortality rate. New imaging techniques and 18F-FDG PET/CT in particular have meant a significant advance in cases of high clinical suspicion and negative or inconclusive echocardiography, increasing the overall sensitivity of the modified Duke criteria.
We report the case of a male patient with prosthetic valve endocarditis, where 18F-FDG PET/CT provided the diagnostic key, determining the origin of the endocarditis and avoiding treatment delay.
A endocardite das próteses valvulares é um desafio diagnóstico real na prática clínica devido à menor sensibilidade dos critérios de Duke modificados e a uma maior percentagem de casos em que o ecocardiograma é negativo ou inconclusivo. O atraso no início do tratamento médico ou cirúrgico aumenta a taxa de morbilidade e mortalidade, de modo que o surgimento de novas técnicas de imagem e, em particular, da 18F-FDG PET/CT tem sido um grande avanço em casos de alta suspeição clínica com ecocardiograma negativo ou duvidoso, o que aumenta a sensibilidade global dos critérios diagnósticos de endocardite.
Apresentamos o caso clínico de um homem com endocardite da prótese valvular em quem 18F-FDG PET/CT foi a chave diagnóstica que permitiu diferenciar a origem da endocardite e evitar o atraso do tratamento.
Infective endocarditis (IE) is a serious endovascular infection that requires early diagnosis because of its high initial mortality and morbidity rates and high risk of complications during follow-up.1,2 It has an estimated incidence of 3.1 to 3.7 episodes per 100000 inhabitants per year, and is especially common in the elderly.3
The clinical presentation of IE can be acute or subacute, and it can develop with cardiac or noncardiac involvement. The most common symptom is fever, followed by anorexia, weight loss, weakness and night sweats. Heart murmurs are detected in 85% of patients and up to 25% suffer embolic complications at the time of diagnosis.4 This wide clinical spectrum makes it difficult to detect, requiring a multidisciplinary approach formed by cardiologists, cardiac surgeons, microbiologists, neurologists and specialists in infectious diseases and imaging. By using newer, recently introduced diagnostic imaging techniques, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in particular, a more accurate diagnosis is now possible.
We present the case of a patient with suspected IE, where this new image fusion technique was key to his management.
Case reportWe report the case of a 54-year-old man with a mechanical aortic prosthetic valve implanted in 1998 following severe aortic regurgitation of rheumatic etiology who came to the emergency department reporting pain, erythema and edema in the left leg, without history of trauma and no fever or dyspnea. He denied recent interventions or dental extractions.
He had hemorrhagic skin lesions on his palms and both soles, most numerous on the left foot, consistent with Janeway lesions. No mobility abnormalities were found, and there was no data to suggest any abdominal infectious process. Auscultation revealed regular heart tones, click of prosthetic aortic valve closure and pansystolic murmur, predominantly in left parasternal border, along with bibasilar crackles.
Blood tests in the emergency department showed an increase in inflammatory parameters (procalcitonin 12.45 ng/ml, C-reactive protein 17.9 mg/dl with neutrophilia).
Portable transthoracic echocardiography (TTE) (Philips CX50, with broadband sector array transducer S5-1 with frequency range 5-1 MHz, Amsterdam, the Netherlands) showed no dysfunction of the mechanical aortic prostheses, but did reveal thickening of the anterior mitral valve above A2 with a nodular image with irregular edges and a maximum diameter of 5 mm, suggesting a mild double mitral lesion, without other relevant changes.
Consecutive blood cultures were positive for multi-drug-susceptible Staphylococcus aureus.
The patient was admitted to the cardiology department with the diagnosis of mitral native valve endocarditis (NVE) secondary to Staphylococcus aureus in a patient with an aortic prosthesis, with probable peripheral embolization. By consensus with the infectious diseases department, the patient was started on antibiotic therapy with cloxacillin, intravenous daptomycin and rifampin.
After developing abdominal and neurological manifestations, abdominal computed tomography (CT) and brain magnetic resonance imaging were performed, showing several foci of splenic and lacunar infarction, with resolution of the symptoms in less than 24 hours.
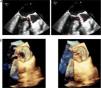
Infectious parameters began to decrease over the initial days of antibiotic treatment. Two-dimensional (2D) and three-dimensional (3D) TTE and transesophageal echocardiography (TEE) showed absence of endocarditis progression and an apparently unaffected, normal-functioning aortic prosthesis, with similar findings as in the initial echocardiography (Figure 1).
Given the high suspicion of aortic prosthetic valve endocarditis (PVE) despite the negative echocardiogram, an 18F-FDG PET/CT was requested because of the high sensitivity and specificity of this technique for diagnosing PVE. To reduce the myocardium physiological uptake of 18F-fluorodeoxyglucose (18F-FDG), the patient was prepared with a high-fat, low-carbohydrate meal and fasted for 18 hours.
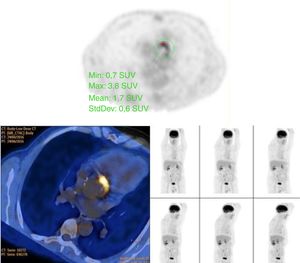
The images revealed an intense hyper-metabolism confined to the aortic annulus, above the aortic-valve prosthesis, with inhibition of the rest of the cardiac metabolism after the prescribed preparation. This confirmed the diagnosis of aortic PVE, with no evidence of pathological deposits of tracer in the mitral valve or other body territories (Figure 2).
18F-fluorodeoxyglucose positron emission tomography/computed tomography reveals intense hyper-metabolism confined to the aortic annulus, above the aortic valve prosthesis, with inhibition of the rest of the cardiac metabolism, with no evidence of pathological deposits of the tracer in other body territories.
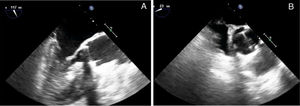
Consequently, repeat 2D and 3D TTE and TEE revealed a previously non-existent, or not visible, 5-6 mm vegetation (Figure 3 and Supplementary Video 1). The vegetation was observed on the posterior surface of the aortic prosthesis and was not causing regurgitation. A nodule persisted on the anterior surface of the mitral valve, but the positron emission tomography/computed tomography (PET/CT) images showed it to be only a simple degenerative finding. No abscesses or other complications of IE were observed and the mechanical aortic prosthesis discs were opening normally, with a mean gradient of 25 mmHg and no significant regurgitation.
(A) 2-dimensional and (B) 3-dimensional transesophageal echocardiography. A 5-6 mm vegetation can be seen on the posterior surface of the aortic prosthesis, with a nodule persisting on the anterior surface of the mitral valve. No abscesses or other complications of infective endocarditis are observed and the mechanical aortic prosthesis discs are opening normally.
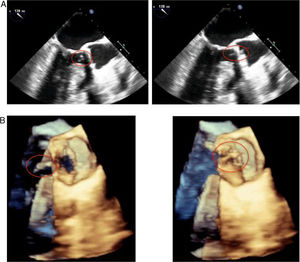
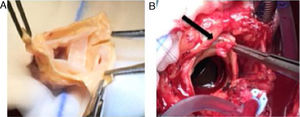
Having confirmed the diagnosis of staphylococcal PVE with the aid of the above images, surgery to replace the prosthesis was considered the most appropriate treatment. The aortic prosthetic valve was replaced by an aortic valve homograft (Figure 4), due to lower risk of reinfection than with mechanical valves thanks to the absence of synthetic components.4
After surgery, antibiotic therapy was maintained for 6 weeks. Postoperative echocardiography showed normal function of the aortic homograft. The patient progressed favorably with negative follow-up blood cultures.
DiscussionMechanical or biological PVE occurs in 1-6% of patients with prosthetic valves, causing 10-30% of all cases of IE.4
Diagnosis is more difficult than in NVE, due to a more atypical clinical presentation and lower sensitivity of the Duke criteria.5,6 As in NVE, the diagnosis of PVE is based on clinical signs and symptoms, blood cultures and imaging techniques. A combination of TTE and TEE is still the main imaging technique used, and it should be performed in all patients with suspicion of IE. However, almost 30% of cases of PVE show normal or inconclusive echocardiographic images, without being able to rule out the diagnosis.4,5,7 As delay in treatment worsens prognosis, other imaging techniques are necessary in cases of high clinical suspicion in order to increase diagnostic sensitivity.
While cardiac CT could be suitable, it only offers anatomical information without providing functional data, which is a major limitation, especially in the case of doubtful structural lesions.7
Nuclear medicine techniques have recently emerged as a supplementary method in patients with suspected endocarditis and uncertain results in other investigations. PET/CT with 18F-FDG in particular has gained importance in recent years.
It measures metabolic tissue activity so it can locate metabolic or functional abnormalities and differentiate them from surrounding healthy tissues. It also provides accurate information about the anatomy thanks to the spatial resolution of CT. The most commonly used radiopharmaceutical, 18F-FDG, is actively incorporated by activated leukocytes, which accumulate at the site of infection.4 Its uptake is proportional to the amount of glucose consumed in a tissue, explaining why this technique is of great use in oncology and infectious diseases. In order to reduce the physiological uptake of 18F-FDG by myocardial tissue, previous preparation with a high-fat, low-carbohydrate meal is required, followed by fasting at least 6 hours.6
While in the case of NVE it does not seem to provide greater diagnostic power,6 several studies report that 18F-FDG PET/CT has higher rates of internal and external validity than echocardiography for PVE8,9; it has demonstrated sensitivity values of 73-85%, with a specificity approaching 80% and a positive predictive value of 85%.6,7 Many authors have proposed adding the abnormal uptake of 18F-FDG in PVE to the modified Duke criteria and this combination has been found to significantly increase sensitivity with little loss of specificity.6 The combination of the modified Duke criteria and 18F-FDG PET/CT would achieve a sensitivity of 97% for early diagnosis of PVE compared to 70% for the criteria alone.7 In a 2015 study, an increase in sensitivity from 52% to 90.7% was calculated.2
There are also other qualities provided by 18F-FDG PET/CT, such as a single acquisition time point of the image, generally at one hour after administration of 18F-FDG. In addition, the utility of 18F-FDG PET/CT in monitoring the response to antimicrobial treatment in patients not selected for cardiac surgery has been reported,1,10 showing an early reduction in the quantified metabolic activity in cases of appropriate antibiotic coverage. However, there is still not enough evidence to support generalized implementation.4 Another advantage of this technique is the ability to detect peripheral septic emboli secondary to IE (intestinal, splenic, etc.),5 although there are certain limitations in the detection of stroke emboli, as the physiological uptake of glucose by the brain is considerable and emboli in this location are usually smaller than 5 mm, which is at the spatial resolution threshold of the current PET/CT scanners.1,5 In our case we did not see any uptake in tissues where peripheral embolization was suspected, probably because the patient was covered by antibiotics at the time the technique was performed.6
Certainly, this imaging technique has other limitations that should not be overlooked. It is important to remember that the heart tissue has physiological uptake of 18F-FDG, so proper preparation has to be ensured before the test. In addition, there are many situations that simulate increased accumulation of this radiopharmaceutical, generating false positives, such as postoperative inflammation in patients who have undergone cardiac surgery in the previous months or certain disorders, like diabetes, the presence of tumors or active thrombi, soft atherosclerotic plaques or vasculitis.5
Following these studies, in the 2015 European Society of Cardiology guidelines, the abnormal uptake of 18F-FDG detected by PET/CT around prostheses implanted for more than three months was considered as a new major criterion in patients with suspected PVE. In this context, an algorithm including echocardiography and 18F-FDG PET/CT was proposed.4
Taking these studies into account, we considered our patient an appropriate candidate for 18F-FDG PET/CT, as echocardiography could not distinguish whether the endocarditis was attributable to the mitral native valve or to the prosthetic aortic valve. In the knowledge that the management of PVE is substantially different from that of NVE, requiring early surgery in the case of Staphylococcus infection, the correct diagnosis was vital to offer to the patient the best treatment.
In conclusion, this case report is a good example to demonstrate the utility of 18F-FDG PET/CT in cases of high suspicion of PVE with negative echocardiogram. This type of image is not a substitute for clinical information, microbiology and echocardiography, but it is a tool to be considered in such patients. Further studies are needed in order to clarify the role of 18F-FDG PET/CT in other contexts of IE and to confirm its value in monitoring antimicrobial treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.