Heart failure with preserved ejection fraction (HFPEF) is a highly prevalent syndrome that is difficult to diagnose in outpatients. The measurement of B-type natriuretic peptide (BNP) may be useful in the diagnosis of HFPEF, but with a different cutoff from that used in the emergency room. The aim of this study was to identify the BNP cutoff for a diagnosis of HFPEF in outpatients.
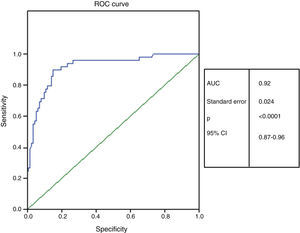
Methods and ResultsThis prospective, observational study enrolled 161 outpatients (aged 68.1±11.5 years, 72% female) with suspected HFPEF. Patients underwent ECG, tissue Doppler imaging, and plasma BNP measurement, and were classified in accordance with algorithms for the diagnosis of HFPEF. HFPEF was confirmed in 49 patients, who presented higher BNP values (mean 144.4 pg/ml, median 113 pg/ml, vs. mean 27.6 pg/ml, median 16.7 pg/ml, p<0.0001). The results showed a significant correlation between BNP levels and left atrial volume index (r=0.554, p<0.0001), age (r=0.452; p<0.0001) and E/E′ ratio (r=0.345, p<0.0001). The area under the ROC curve for BNP to detect HFPEF was 0.92 (95% confidence interval: 0.87-0.96; p<0.001), and 51 pg/ml was identified as the best cutoff to detect HFPEF, with sensitivity of 86%, specificity of 86% and accuracy of 86%.
ConclusionsBNP levels in outpatients with HFPEF are significantly higher than in those without. A cutoff value of 51 pg/ml had the best diagnostic accuracy in outpatients.
Insuficiência cardíaca com fração de ejeção preservada (ICFEP) é uma síndrome de alta prevalência e difícil diagnóstico no ambulatório. O doseamento de peptídeo natriurético tipo B (BNP) pode ser útil no diagnóstico de ICFEP, porém com valor de corte diferente daquele utilizado na sala de emergência. O objetivo desse estudo foi identificar o ponto de corte do BNP em doentes ambulatórios para diagnóstico de ICFEP.
Métodos/resultadosEstudo prospectivo observacional envolvendo 161 doentes ambulatórios (68,1 ± 11,5 anos, 72% mulheres) com suspeita de ICFEP. Doentes foram submetidos a exame clínico, eletrocardiograma, ecocardiograma com Doppler tecidual e doseamento de BNP e classificados de acordo com critérios propostos por Paulus et al. para diagnóstico de ICFEP. ICFEP foi confirmada em 49 doentes que apresentavam valores mais elevados de BNP (144,4 pg/mL mediana 113 pg/mL versus 27,6 pg/mL mediana 16,7 pg/mL p < 0,0001). Uma correlação significativa foi mostrada entre BNP e volume da aurícula esquerda indexada (rho = 0,554, p < 0,0001), idade (rho = 0,452, p < 0,0001) e relação E/E’ (rho = 0,345, p < 0,0001). A área sob a curva ROC para BNP detectar ICFEP foi 0,92 (95% IC, 0,87-0,96, p < 0,0001) e o valor de corte de 51 pg/mL foi o que melhor se correlacionou com o diagnóstico de ICFEP (sensibilidade 86%, especificidade 86%, acuidade de 86%).
ConclusãoValores de BNP em doentes ambulatórios com ICFEP são significativamente mais elevados que os valores dos doentes sem ICFEP. O valor de corte do BNP de 51 pg/mL apresentou melhor acuidade diagnóstica para ICFEP em doentes ambulatórios.
Heart failure (HF) is a major public health problem worldwide – one in five people aged over 40 will develop HF at some stage – and in Brazil it is the leading cause of hospitalization in those aged over 60.1
Over half of these patients are classified as having HF with preserved ejection fraction (HFPEF)2; this proportion is growing in both developed and developing countries due to ageing populations and increased awareness among physicians of the syndrome, which predominantly affects women and those with multiple comorbidities.3–5 The costs associated with HF are high, for both hospitalization and outpatient care, irrespective of left ventricular ejection fraction (LVEF). A simple exam would thus be useful for screening these patients with a view to more thorough investigation.2–6
B-type natriuretic peptide (BNP) is released in response to increased left ventricular (LV) filling pressure7 and end-diastolic wall stress,8 and plasma BNP levels can help in both diagnosis (confirming or excluding HF) and prognostic assessment of HF.9,10
Paulus et al.6 proposed criteria for the diagnosis of HFPEF that included Doppler echocardiographic parameters and BNP measurement. The BNP cutoff used was based on data from a study by Maisel et al. of patients admitted to the emergency room with acute HF.11
However, BNP assessment in outpatients show lower values than those suggested by Paulus et al. for a diagnosis of HFPEF, although with different Doppler echocardiographic criteria for diagnostic confirmation.12,13
The use of the BNP cutoff proposed by Paulus et al. has not been validated in clinical practice for outpatients, but BNP measurement, an inexpensive exam, may help to exclude HFPEF and thus direct investigation of a patient's symptoms to other areas.
The aim of this study was to determine the diagnostic accuracy of BNP measurement and the best cutoff to confirm or exclude HFPEF in outpatients using the criteria proposed by Paulus et al.
MethodsPopulationThis prospective, observational, cross-sectional study enrolled 161 consecutive outpatients (mean age 68.1±11.5 years; 72% female) with suspected HF. All were in New York Heart Association (NYHA) functional class II or III. HFPEF was defined in accordance with the European Society of Cardiology (ESC) criteria, which include signs or symptoms of HF, LVEF >50%, LV end-diastolic volume index <97 ml/m2 and diastolic LV dysfunction.6
Diagnostic evidence of diastolic dysfunction was obtained by tissue Doppler echocardiography showing an E/E’ ratio of >15. According to the ESC guidelines, if the E/E’ ratio is suggestive of diastolic dysfunction (8-15), other echocardiographic measures should be used to confirm the diagnosis such as LV mass index (>122 and >149 g/m2 for women and men, respectively), left atrial volume index (LAVI) (>40 ml/m2), and E/A ratio <0.5 with E deceleration time >280 ms. An electrocardiogram showing atrial fibrillation with an E/E’ ratio of 8-15 also confirms the diagnosis of HFPEF.6
Patients with severe valve disease, permanent pacemaker or chronic obstructive pulmonary disease, as well as those who had undergone cardiac surgery in the previous six months, were excluded. The study was conducted in accordance with the Helsinki declaration and the protocol was approved by the institution's ethics committee (no. 00410.258.000-08); all patients gave their written informed consent to inclusion in the study.
Conventional and tissue Doppler echocardiographyDoppler echocardiography was performed on a VIVID 7 ultrasound system (GE®, USA), and the images analyzed in EchoPac software by an experienced investigator blinded to the results of other exams. The exam was carried out in accordance with the recommendations for chamber quantification of the American Society of Echocardiography/European Association of Echocadiography.14
Systolic function was assessed by estimation of LVEF and longitudinal strain (S’) by tissue Doppler.
LAVI was calculated by Simpson's biplane method based on apical 2- and 4-chamber views in LV end-systole, indexed to body surface area. Diastolic function was estimated on the basis of the mean of five consecutive cycles. Early (E) and late (A) transmitral flow and E-wave deceleration time were measured. Myocardial relaxation velocity at beginning diastole (E’) was measured by tissue Doppler at the septal and lateral segments of the mitral annulus and the mean used in the analysis. All exams were recorded in digital form for subsequent analysis and review.
ElectrocardiographyAll patients underwent 12-lead resting ECG to screen for atrial fibrillation.
B-type natriuretic peptidePlasma BNP was measured by the Triage BNP test (Biosite USA), a rapid fluorescence immunoassay for quantitative BNP measurement using the Triage meter. BNP values were expressed in pg/ml.
Statistical analysisThe statistical analysis was performed using SPSS® version 15.0. Continuous variables with normal distribution were expressed as means ± standard deviation, and the remainder as medians. ANOVA and the chi-square test were used to determine differences in means between the continuous variables with normal distribution, between those with abnormal distribution and between categorical variables, respectively. Spearman's correlation (r) was used to measure the association between BNP levels and clinical and echocardiographic variables. A receiver-operator characteristic (ROC) curve was constructed to determine the sensitivity and specificity of BNP for a diagnosis of HFPEF. A level of statistical significance of 0.05 was adopted.
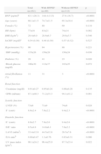
ResultsThe demographic, clinical, laboratory and echocardiographic characteristics of the study population are shown in Table 1, from which it can be seen that patients with HFPEF were older, and had a higher prevalence of hypertension, diabetes and atrial fibrillation and lower glomerular filtration rate (GFR) than those without HFPEF. LV function assessed by Doppler echocardiography showed more marked systolic and diastolic dysfunction in the group with HFPEF.
Demographic, clinical and laboratory characteristics of the study population, and systolic and diastolic function by Doppler echocardiography and tissue Doppler.
| Total (n=161) | With HFPEF (n=49) | Without HFPEF (n=112) | p | |
| BNP (pg/ml)a | 63.1 (28.3) | 144.4 (113) | 27.6 (16.7) | <0.0001 |
| Age (years) | 68.1±11.5 | 74.7±11.5 | 65.3±10.4 | <0.0001 |
| Female (%) | 72 | 80 | 69 | 0.110 |
| HR (bpm) | 77±16 | 83±21 | 74±14 | 0.002 |
| BMI (kg/m2) | 29.4±6.0 | 28.9±6.7 | 29.6±5.7 | 0.546 |
| hsCRP (mg/dl)a | 0.54 (0.30) | 0.48 (0.30) | 0.57 (0.32) | 0.527 |
| Hypertension (%) | 90 | 94 | 88 | 0.221 |
| SBP (mmHg) | 153±26 | 159±28 | 150±24 | 0.036 |
| Diabetes (%) | 29 | 41 | 23 | 0.020 |
| Blood glucose (mg/dl) | 106±30 | 112±37 | 103±25 | 0.072 |
| Atrial fibrillation (%) | 11 | 31 | 3 | <0.0001 |
| Renal function | ||||
| Creatinine (mg/dl) | 0.91±0.27 | 0.95±0.24 | 0.88±0.28 | 0.135 |
| GFR (ml/min) | 87.1±40.3 | 71.2±33.3 | 94.1±41.2 | 0.001 |
| Systolic function | ||||
| LVEF (%) | 73±8 | 71±9 | 74±8 | 0.028 |
| S’ (cm/s) | 8.9±2.4 | 7.6±2.2 | 9.4±2.3 | <0.0001 |
| Diastolic function | ||||
| E’ (cm/s) | 8.9±2.7 | 7.8±2.6 | 9.4±2.6 | <0.0001 |
| E/E’ ratio | 9.5±4.8 | 14.0±6.1 | 7.6±2.2 | <0.0001 |
| LAVI (ml/m2) | 33.4±12.0 | 44.5±12.8 | 28.5±7.6 | <0.0001 |
| E/A ratiob | 0.88±0.47 | 1.1±0.76 | 0.83±0.31 | 0.006 |
| LV mass index (g/m2) | 90.1±24.2 | 96.4±25.0 | 87.7±23.4 | 0.022 |
BMI: body mass index; BNP: B-type natriuretic peptide; GFR: glomerular filtration rate; HR: heart rate; hsCRP: high sensitivity C-reactive protein; LAVI: left atrial volume index; LV: left ventricular; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure. Categorical variables – Pearson's chi-square; Numerical variables – ANOVA; a median; b not assessed in patients with atrial fibrillation (n=143). Significant differences between the groups for p<0.05.
BNP levels were significantly different between those with and without HFPEF (mean 144.4 pg/ml, median 113 pg/ml vs. mean 27.6 pg/ml, median 16.7 pg/ml, respectively, p<0.0001).
Table 2 shows the correlations between BNP and clinical and echocardiographic variables. There was a direct correlation between BNP and LAVI, age and E/E’ ratio and an indirect correlation with estimated GFR.
Correlation between BNP and clinical, laboratory and Doppler echocardiographic variables.
| Spearman (r) | p | |
| Age (years) | 0.452 | <0.0001 |
| SBP (mmHg) | -0.012 | 0.880 |
| HR (bpm) | 0.045 | 0.570 |
| GFR (ml/min) | -0.329 | <0.0001 |
| LVEF (%) | 0.049 | 0.535 |
| S’ (cm/s) | -0.203 | 0.011 |
| E’ (cm/s) | -0.147 | 0.062 |
| E/E’ ratio | 0.345 | <0.0001 |
| LAVI (ml/m2) | 0.554 | <0.0001 |
| E/A ratio | -0.077 | 0.363 |
| LV mass index (g/m2) | 0.059 | 0.455 |
BNP: B-type natriuretic peptide; GFR: glomerular filtration rate estimated by the Cockcroft-Gault formula; HR: heart rate; LAVI: left atrial volume index; LV: left ventricular; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure. Significant correlation with p=0.05.
The area under the ROC curve for BNP to detect HFPEF was 0.92 (95% confidence interval [CI] 0.87-0.96, p<0.0001) (Figure 1), and 51 pg/ml was the most appropriate cutoff to detect HFPEF, with sensitivity of 86%, specificity of 86%, positive predictive value of 93%, negative predictive value of 72%, and diagnostic accuracy of 86%.
DiscussionThis is the first prospective study to use the ESC criteria6 for the diagnosis of HFPEF in outpatients and to demonstrate that HFPEF patients present high BNP values, although lower than those found in emergency room patients. Our results suggest that BNP cutoffs for a diagnosis of HFPEF in outpatients may be lower than those in the ESC consensus statement, which was based on studies in patients presenting with acute HF.6
In the emergency room, signs and symptoms of congestive HF are usually present and since many patients are hospitalized due to acute pulmonary edema, BNP levels are higher than in outpatients, as demonstrated in a study of 1586 emergency room patients, in whom a BNP cutoff of 100 pg/ml had sensitivity of 90% and specificity of 76%, irrespective of LVEF, for differentiating acute HF from other causes of dyspnea.15
Exertional dyspnea may be the only symptom in out-patients with HFPEF, with no sign of congestion, and those most commonly affected are women, the elderly, the obese, and those with multiple comorbidities.16 The diagnosis of HFPEF in such cases is a challenge to physicians and a biomarker such as BNP may be useful in identifying these patients, but based on lower BNP levels than in emergency room patients.
Our results show that patients with HFPEF present significant diastolic dysfunction, characterized by alterations in ventricular relaxation (E’) and increased LV filling pressures (as shown by LAVI and E/E’ ratio) but normal LVEF. The correlation observed between BNP values and echocardiographic parameters reflects the association between BNP and LV diastolic function, and BNP measurement is thus a useful and simple exam to assess dyspnea in outpatients with suspected HF.
Arques et al.17 studied 26 outpatients presenting chronic dyspnea as the only manifestation of HF who underwent cardiac catheterization, which is considered the gold standard exam to confirm a diagnosis of HFPEF.18 The study showed that BNP of 31 pg/ml was predictive of HFPEF (p=0.003), with 67% sensitivity and 73% specificity (area under the ROC curve 0.76, 95% CI: 0.55-0.90; p=0.007).17
Penicka et al.19 assessed 30 outpatients (73% female) with unexplained chronic dyspnea, in NYHA functional class II or III and LVEF >50%, who underwent cardiac catheterization. HFPEF was defined as LV end-diastolic pressure of >16 mmHg at rest during hemodynamic study, and HFPEF was confirmed in 20 of these patients (66%). BNP was higher in the HFPEF group than in controls (68 pg/ml vs. 21 pg/ml, p=0.09), but only three patients had BNP levels above the cutoff recommended by Paulus et al. (200 pg/ml) for a diagnosis of HFPEF.19
Kitzman et al.13 assessed 147 outpatients, of whom 59 had HFPEF; mean BNP in this group was 56±30 pg/ml.
Our findings are consistent with the results of the above studies13,17,19 in terms of BNP cutoff points in patients with HFPEF, and confirm that outpatients with HFPEF can have BNP levels well below the cutoffs established by the ESC.6
Determining a more appropriate BNP cutoff than those currently used could help in screening outpatients with suspected HFPEF. Zuber et al. studied 384 primary care patients to assess the accuracy of BNP for diagnosing HF as determined by clinical and echocardiographic data and found that 31 (8%) had HFPEF and 193 (50%) had systolic HF with reduced ejection fraction. The recommended BNP cutoff of 100 pg/ml was used to diagnose or exclude HF, which gave a false negative result in 25% of patients with HF. The study concluded that this cutoff failed to exclude HF in primary care patients.20
The latest ESC guidelines for the diagnosis and treatment of acute and chronic HF21 propose a new BNP cutoff of 35 pg/ml to exclude HF (with reduced or preserved ejection fraction) in outpatients, which may reduce the number of false negatives and unnecessary echocardiographic exams. This cutoff was based on data from eight studies22–29 that analyzed patients with suspected HF or at risk of developing HF due to ventricular dysfunction. The cutoff of 51 pg/ml determined in our study is specific to HFPEF and showed a positive predictive value of 93% and negative predictive value of 72%, making it useful to confirm or exclude HFPEF in outpatients.
In view of the ageing of populations, which will increase the number of cases of HFPEF, BNP measurement could be a less costly and simpler way to screen outpatients for suspected HF than echocardiography.
Certain limitations of our study should be borne in mind. As the absolute number of patients in whom HFPEF was confirmed was small, and given the heterogeneous nature of the syndrome, and the fact that comorbidities can effect BNP levels, further studies involving large samples of outpatients will be required to validate our findings.
ConclusionsBNP levels in outpatients with HFPEF, as diagnosed by ESC criteria, are higher than in those without HFPEF. In the study population, a cutoff of 51 pg/ml had the best diagnostic accuracy to identify HFPEF in outpatients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Lagoeiro Jorge AJ, Di Calafiori Freire M, Ribeiro ML, et al. Utilidade do doseamento do peptídeo natriurético tipo B em doentes ambulatórios com insuficiência cardíaca com fração de ejeção preservada. Rev Port Cardiol. 2013;32:647–652.