We report a case of very late bare-metal stent restenosis, in which assessment by two intracoronary imaging techniques (intravascular ultrasound and optical coherence tomography) revealed the underlying mechanism (neoatherosclerosis) and facilitated percutaneous treatment (direct bare-metal stent-in-stent). We also take the opportunity to briefly describe the advantages and limitations of both techniques in this pathology.
Neste artigo fazemos referência a um caso de reestenose muito tardia de um stent convencional que, através de duas técnicas de diagnóstico intracoronário, ultrassonografia intravascular (IVUS) e tomografia de coerência ótica (OCT), permite dar a conhecer o seu mecanismo subjacente (neoaterosclerose) e facilita o seu tratamento percutâneo (stent convencional direto intrasent). Aproveitamos também a ocasião para fazer uma breve descrição das vantagens e limitações de ambas as técnicas neste tipo de patologia.
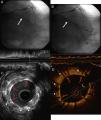
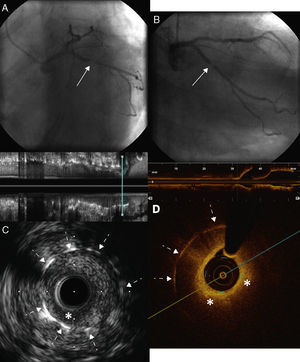
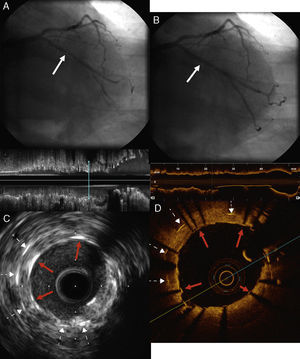
A 73-year-old man with a history of permanent atrial fibrillation under anticoagulation therapy and chronic ischemic heart disease, revascularized in 2002 with two bare-metal stents (BMS) in the circumflex artery (Cx), was referred for coronary angiography in 2013 for unstable angina. This revealed severe focal in-stent restenosis (ISR) of the BMS implanted in the proximal segment of the Cx (Figure 1A and 1B). In order to elucidate the causal mechanism and type of ISR, the Cx was examined using intravascular ultrasound (IVUS), which showed focal ISR with a hypoechogenic and heterogenous image of the adventitia. Further investigation of the ISR by optical coherence tomography (OCT) showed neoatherosclerosis with an image suggestive of a lipid plaque at the level of the ISR, hypointense and highly attenuated, making it difficult to visualize the stent struts (Figure 1D). In view of these findings, we opted for direct stent-in-stent placement of a BMS (3.0 mm × 12 mm at 16 atm) (Figure 2A). Angiography (Figure 2B) and IVUS and OCT (Figure 2C and 2D) confirmed correct stent expansion and apposition.
(A and B) Coronary angiography showing severe intrastent restenosis at the level of the proximal circumflex artery (solid arrows) with distal TIMI 3 flow; (C) intravascular ultrasound images of the restenosis showing heterogeneous tissue with hypoechogenic areas on the surface (asterisk) and correct expansion of the stent implanted in 2002, with no sign of fracture (dotted arrows); (D) optical coherence tomography images of the restenosis showing neointima with a lipid appearance surrounded by a hyperintense fibrous capsule and consisting of hypointense tissue with poorly defined borders and high attenuation (asterisks) producing a shadow that restricts visualization of the stent struts (dotted arrows).
(A) Bare-metal stent-in-stent placement in the restenosis (solid arrow); (B) angiographic result of direct stent placement, with no significant residual stenosis (solid arrow); (C and D) intravascular ultrasound and optical coherence tomography images of the newly implanted stent, showing correct expansion and complete apposition of all struts (solid red arrows), together with struts of the stent implanted in 2002 (dotted arrows).
Percutaneous treatment of ISR has traditionally been considered a challenge due to the characteristics of the underlying neointimal hyperplasia, which increases resistance to dilation and is liable to recur.1 However, the recent discovery that neoatherosclerosis is the substrate responsible for a significant proportion of ISR has reawakened interest in its etiological mechanisms and possible treatment.2 Neoatherosclerosis, characterized by atherosclerotic alterations such as calcium deposits, lipid cores and cholesterol crystals in the neointima that covers both BMS and drug-eluting stents (DES), is at least partly caused by the inflammation and endothelial dysfunction in the vessel wall associated with both types of stent. These characteristics mean that plaque rupture and exposure of its highly prothrombotic contents is responsible for a large proportion of late stent thrombosis.2–5
There is general recognition of the diagnostic value of OCT and IVUS in quantifying the percentage, extent and precise location of ISR (edges, proliferation, etc.), and detecting mechanical factors that may be implicated in its development such as stent underexpansion or fracture.1 As seen in this case report, detection of a high lipid content in ISR is an indication for direct stent placement without predilation, thereby reducing the risk of plaque rupture or embolization, future restenosis and/or revascularization due to dissection of stent edges caused by balloon displacement during inflation, and avoiding the expense of using one or more balloon types (compliant, noncompliant, cutting, etc.).
Although DES are reported to provide better clinical and angiographic results than BMS in percutaneous treatment of ISR,1 their performance in ISR caused by neoatherosclerosis has not been studied. It is however known that neoatherosclerosis occurs earlier and more frequently with DES,2,5 which need a longer period of dual antiplatelet therapy. For this reason, and because our patient required anticoagulant therapy, it was decided to implant a new BMS stent-in-stent, and a good result was confirmed by both IVUS and OCT, with no need for postdilation.
As can be seen in the figures, far from being mutually exclusive alternatives, each of these two imaging techniques has both advantages and limitations. The higher spatial resolution of OCT enables better visualization and characterization of the tissue causing ISR, but the attenuation caused by the necrotic core produces a shadow that obscures the stent struts at this level and can conceal stent underexpansion or fracture that may be responsible for the ISR. Furthermore, assessment of ISR by OCT requires the blood to be flushed from the arterial lumen, and image quality may be impaired when occlusive or ostial ISR prevents effective flushing. The greater penetration of IVUS provides a more accurate assessment and measurement of the entire arterial wall, and since there is no attenuation by the necrotic core, all the struts can be visualized.
In conclusion, this case illustrates the central role of intracoronary imaging techniques in the diagnosis and characterization of ISR, as well as in planning and guiding percutaneous treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ruiz-García J, Sánchez-Recalde Á, Jiménez-Valero S, et al. Utilidade prática das técnicas de diagnóstico intracoronário no tratamento percutâneo da reestenose intrastent. Rev Port Cardiol. 2013;32:1019–1022.