The interventional cardiologist chooses a specific stent type based on the risk-benefit profile for each case. In general, drug-eluting stents should be considered in all clinical conditions, except if there are concerns or contraindications for prolonged dual antiplatelet therapy. The aim of this work was to describe the use of bare-metal vs. drug-eluting stents in patients undergoing percutaneous coronary intervention (PCI) after an acute coronary syndrome in Portuguese hospitals, according to patients’ demographic and clinical characteristics and institutional characteristics.
MethodsWithin the EURopean Hospital Benchmarking Processes (EURHOBOP) study, we retrospectively assessed 3009 consecutive patients in 10 Portuguese hospitals in 2009. Only patients with stents implanted during PCI (n=1194) were analyzed.
ResultsA total of 425 patients (36%) received a bare-metal stent and 769 patients (64%) received a drug-eluting stent. A history of previous PCI, current non-ST-elevation myocardial infarction, anterior descending artery as the infarct-related artery and being initially admitted to a hospital with a catheterization laboratory were associated with drug-eluting stent implantation. Age under 45 or over 80, anemia and previous anticoagulation and/or atrial fibrillation were associated with bare-metal stent use.
ConclusionsApproximately two-thirds of patients received drug-eluting stents, which were less frequently implanted in patients with ST-elevation myocardial infarction, aged over 80 years, female, with a previous history of stroke, anticoagulation and/or atrial fibrillation and anemia. Patients who had previously undergone PCI and those with the anterior descending artery as the infarct-related artery were more likely to receive a drug-eluting stent.
O tipo de stent é selecionado com base numa análise de risco-benefício individual. Em geral, os stents revestidos devem ser considerados, exceto se existirem preocupações ou contraindicações para a terapêutica antiplaquetária dupla. O objetivo deste estudo foi descrever a utilização de stents metálicos versus revestidos em doentes submetidos a angioplastia após síndrome coronária aguda em hospitais Portugueses, de acordo com características demográficas e clínicas dos doentes, e institucionais.
MétodosNo estudo EURHOBOP, em 3009 doentes internados consecutivamente em 10 hospitais portugueses por síndrome coronária aguda, 1194 foram submetidos a implantação de stent durante intervenção coronária percutânea.
ResultadosUm total de 425 doentes (36%) receberam um stent metálico e 769 (64%) receberam um stent revestido. Verificamos que doentes com uma história prévia de intervenção coronária percutânea, com síndrome coronária aguda sem elevação do segmento-ST, intervencionados na artéria descendente anterior e admitidos num hospital com laboratório de hemodinâmica mais frequentemente receberam stent revestido. Contudo, um stent metálico foi mais frequentemente usado quer em doentes jovens (<45 anos) quer muito idosos (mais de 80 anos), anémicos e com uma história prévia de anticoagulação e/ou fibrilhação auricular.
ConclusõesAproximadamente dois terços dos doentes receberam um stent revestido, menos frequentemente em enfarte com elevação do segmento ST, idade superior a 80 anos, mulheres ou história prévia de acidente vascular cerebral, anticoagulação e/ou fibrilhação auricular ou anemia. Doentes com história prévia de intervenção coronária percutânea e com enfarte no território da artéria descendente anterior tinham mais probabilidade de receber um stent revestido.
Treatments for acute coronary syndrome (ACS) have improved considerably in the last 30 years and there are currently several approaches available for revascularization, including fibrinolysis, percutaneous coronary intervention (PCI), coronary artery bypass surgery and pharmacologic therapy.1 Factors like previous medical history and disease presentation (as unstable angina, non-ST-elevation myocardial infarction or ST-elevation myocardial infarction [STEMI]), angiographic findings and issues concerning both co-adjuvant and secondary prevention therapy (particularly compliance with and safety of dual antiplatelet therapy) may influence the choice of strategy for reperfusion and definitive revascularization.2
The increasing use of PCI over the last decade is based on studies that support the effectiveness of this approach in securing and maintaining coronary artery patency, in particular avoiding some of the bleeding risks of fibrinolysis.3–6 The reduction of restenosis in the target lesion by 60%–70% when drug-eluting stents (DES) are used instead of bare-metal stents (BMS) has also contributed to the exponential growth of PCI for revascularization of patients with coronary disease.7–10 Many randomized controlled trials have documented that primary PCI is superior to intravenous thrombolysis for the treatment of STEMI, thus contributing to a growing trend for the use of PCI in STEMI patients.11 In patients with non-ST-elevation ACS (NSTE-ACS), risk stratification should be performed as early as possible to identify high-risk individuals. Only high-risk patients with NSTE-ACS benefit from an early invasive approach such as PCI.12 In Portugal, according to the Portuguese Registry of ACS, use of PCI rose from 14.8% and 24.9% in 2002 to 50.2% and 38.3% in 2008 for patients presenting with STEMI and NSTE-ACS, respectively.13
Currently, the interventional cardiologist chooses a specific stent type based on the risk-benefit profile for each case. In general, DES should be considered in all clinical conditions and lesion subsets, except if there are concerns or contraindications for prolonged dual antiplatelet therapy.14 There are particular situations in which the use of DES is strongly recommended, including in the presence of left main artery disease, diabetes, saphenous vein grafts, small vessels (<2.5 mm diameter), long lesions, bifurcations, multiple lesions and in-stent restenosis.2 Besides clinical considerations, it is important to note that DES were two or three times more expensive than BMS in the recent past and this factor may influence the choice of stent used in clinical practice.
Therefore, the aim of this work was to describe the use of BMS vs. DES in patients with ACS undergoing PCI in routine practice in Portuguese hospitals, according to patients’ demographic and clinical characteristics and institutional characteristics.
MethodsPatient data were collected in the framework of the EURopean HOspital Benchmarking by Outcomes in acute coronary syndrome Processes (EURHOBOP) project, which was a multicenter and multinational retrospective study of patients diagnosed with ACS consecutively discharged from 70 European hospitals (in Finland, France, Germany, Greece, Italy, Portugal and Spain). This study only considers patients admitted to the 10 Portuguese hospitals.
Portuguese hospitalsData from public hospitals, from North to South and East to West of the country, serving both urban and rural populations and with different levels of specialization (with or without a catheterization laboratory or cardiac surgery department), were included. Participating hospitals are listed in the Acknowledgments section. Of the 10 Portuguese hospitals, five had a catheterization laboratory and only three had a cardiac surgery department. The number of beds ranged from 280 to 1124.
Study participantsFrom each hospital, retrospective data on 300 consecutive patients from the year 2009 were collected. In hospitals where the annual number of cases was insufficient to obtain the 300-patient sample, the recruitment period was extended back to 2008.
A total of 3009 ACS patients were included in the final sample. In this study, patients without PCI or stent implantation during PCI, and patients with PCI but with missing data on stent type implanted (n=1793), were excluded, as were all patients who had both types of stent (DES and BMS) implanted during the same episode (n=22) (Figure 1).
Data collectionData were collected by trained medical record extractors using a standardized data collection form. The main source of information was the discharge letter, however information on emergency room records and laboratory information systems were also accessed whenever available. Information was extracted on the type of diagnosis, demographic characteristics, previous medical history, clinical and laboratory admission data, procedures used during hospitalization, severity indicators and complications during hospitalization. Information on age, gender, main hospital stay characteristics and vital status was available for all patients. Since information collected on previous medical history is highly relevant for treatment and prognosis, we assumed that information on previous coronary heart disease and cardiovascular risk factors was reported in the files. Therefore, when nothing was stated in the records regarding these issues, we assumed they did not exist. Patients with non-classifiable ACS type due to either subacute presentation or left bundle branch block of unknown duration or with missing data on this variable were included in the overall analysis, but excluded from the analysis of the type of stent use according to the type of diagnosis.
Statistical analysisDescriptive statistics were used to characterize the patients included in this study. The chi-square test or Fisher's exact test, when applicable, were used to compare the characteristics of patients implanted with BMS or DES. Multivariate logistic regression was used to estimate odds ratios for the association of patient and hospital stay characteristics with the stent type used during PCI. All variables were initially included in the model. Age and gender were forced to stay, regardless of their effect in this sample. Variables were then removed that had no significant association with the stent type implanted (BMS or DES) and no confounding role in the effect of other predictors (based on a change of over 10% in the regression coefficients), one at a time, until the final model. Initially patients with STEMI and NSTE-ACS were analyzed separately, but since the determinants of stent type used were not significantly different, as assessed by interaction terms, it was decided to analyze all ACS together, including the type of ACS as an additional covariable.
STATA version 12.0 (Stata Corporation, College Station, Texas, USA) was used for data analysis and a p value <0.05 was considered statistically significant.
EthicsThe ethics committee of the University of Porto Medical School and the National Commission for Data Protection approved the study. These two entities agreed that it would not be necessary to ask for patients’ informed consent, since the study was based on the collection of retrospective clinical data from medical records during hospitalization, and the confidentiality of patients’ identification was assured.
ResultsThe 1194 consecutive patients with stent implantation had a mean age of 64 (standard deviation 13) years and three-quarters were men. One-third were smokers, one-third had diabetes and two-thirds had a history of hypertension. A total of 425 patients (36%) received a BMS, while 769 patients (64%) received a DES.
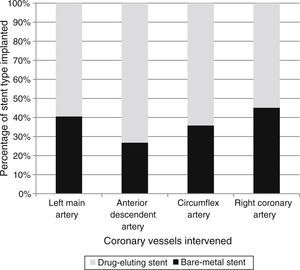
In univariate analysis, those with previous PCI and male gender had higher rates of DES implantation, while BMS had been chosen more frequently in older patients, in patients with previous history of stroke, previous anticoagulation and/or atrial fibrillation, and anemia (Table 1). In STEMI patients, BMS were used more frequently than DES (61.5% vs. 47.9%, p<0.001). Patients in whom the left anterior descending artery (LAD) was treated had a higher rate of DES implantation, whereas a higher rate of BMS implantation was seen in the right coronary artery (Figure 2).
Baseline clinical characteristics of patients undergoing percutaneous coronary intervention with stent implantation, according to type of stent used.
| Overall | Stent type | |||
|---|---|---|---|---|
| n (%) | BMS, n (%) | DES, n (%) | p | |
| Demographic and clinical characteristics | ||||
| Age, years | ||||
| <45 | 102 (8.5) | 38 (8.9) | 64 (8.3) | <0.001 |
| 45–79 | 987 (82.7) | 309 (72.7) | 678 (88.2) | |
| ≥80 | 105 (8.8) | 78 (18.4) | 27 (3.5) | |
| Gender | ||||
| Male | 887 (74.3) | 301 (70.8) | 586 (76.2) | 0.042 |
| Female | 307 (25.7) | 124 (29.2) | 183 (23.8) | |
| Previous medical history | ||||
| Smoking | 398 (33.3) | 127 (29.9) | 271 (35.2) | 0.060 |
| Diabetes | 317 (26.6) | 100 (23.5) | 217 (28.2) | 0.079 |
| Hypertension | 767 (64.2) | 276 (64.9) | 491 (63.9) | 0.706 |
| Myocardial infarction | 191 (16.0) | 64 (15.1) | 127 (16.5) | 0.511 |
| Stroke | 55 (4.6) | 28 (6.6) | 27 (3.5) | 0.015 |
| PCI | 124 (10.4) | 29 (6.8) | 95 (12.4) | 0.003 |
| CABG | 44 (3.7) | 17 (4.0) | 27 (3.5) | 0.668 |
| Heart failure | 49 (4.1) | 17 (4.7) | 29 (3.8) | 0.436 |
| Anticoagulation and/or AF | 54 (4.5) | 35 (8.2) | 19 (2.5) | <0.001 |
| Renal failurea | 69 (5.8) | 30 (7.1) | 39 (5.1) | 0.159 |
| Alzheimer's disease | 9 (0.8) | 3 (0.7) | 6 (0.8) | 0.594 |
| Anemiab | 227 (19.0) | 102 (24.0) | 125 (16.3) | 0.001 |
| Characteristics of current ACS | ||||
| Type of ACS | ||||
| STEMI | 613 (52.7) | 252 (61.5) | 361 (47.9) | <0.001 |
| NSTE-ACS | 550 (47.3) | 158 (38.5) | 392 (52.1) | |
| In-hospital characteristics | ||||
| Catheterization | 756 (63.3) | 258 (60.7) | 498 (64.8) | 0.164 |
| In-hospital outcomes | ||||
| Length of hospital stay, daysc | 6 (4–8) | 6 (4–9) | 5 (4–7) | <0.001 |
| Vital status | ||||
| Alive | 1162 (97.3) | 402 (94.6) | 760 (98.8) | <0.001 |
| Dead | 32 (2.7) | 23 (5.4) | 9 (1.2) | |
ACS: acute coronary syndrome; AF: atrial fibrillation; BMS: bare-metal stent; CABG: coronary artery bypass grafting; DES: drug-eluting stent; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
In multivariate analysis (Table 2), DES use was more frequent in ACS patients with previous PCI (odds ratio [OR] 2.02), LAD treated (OR 2.58), in those initially admitted to a hospital with a catheterization laboratory (OR 1.40) and NSTE-ACS patients (OR 1.80). Age under 45 (OR 0.63) or over 80 (OR 0.17), anemia (OR 0.56), and previous anticoagulation and/or atrial fibrillation (OR 0.25) were associated with a lower likelihood of DES use.
Independent predictors of the use of drug-eluting stents in acute coronary syndrome patients.
| Adjusted OR | 95% CI | |
|---|---|---|
| Age, years | ||
| <45 | 0.63 | 0.40–0.98 |
| 45–79 | 1 | |
| ≥80 | 0.17 | 0.10–0.28 |
| Female gender (vs. male) | 0.84 | 0.61–1.15 |
| Previous percutaneous coronary intervention (vs. not) | 2.02 | 1.24–3.28 |
| Anemiaa(vs. not) | 0.58 | 0.41–0.82 |
| Previous anticoagulation and/or AF (vs. not) | 0.25 | 0.13–0.48 |
| NSTE-ACS (vs. STEMI) | 1.78 | 1.35–2.36 |
| LAD treated (vs. not) | 2.58 | 1.97–3.38 |
| Catheterization in the hospital (vs. not) | 1.40 | 1.05–1.86 |
AF: atrial fibrillation; CI: confidence interval; LAD: left anterior descending artery; NSTE-ACS: non-ST-elevation acute coronary syndrome; OR: odds ratio; STEMI: ST-elevation myocardial infarction.
This study involved a retrospective review of the medical records and discharge letters of a large sample of ACS patients. It enabled us to generate an insightful overview of clinical patterns of stent use in the routine care of patients diagnosed with ACS who underwent elective or primary PCI in 10 Portuguese hospitals.
Overall, 65% of patients who were diagnosed with STEMI or NSTE-ACS were implanted with a DES. This frequency is similar to that in other observational studies examining the use of DES and BMS in patients undergoing PCI across Europe, particularly in Mediterranean countries.15 Ramcharitar et al. showed the highest frequency of DES use in northern Europe (69.3%), followed by western Europe (64.2%), Mediterranean countries (60.4%) and central Europe (20.1%) for the period 2005–2006.15 In the same period, DES use in patients with STEMI in seven countries ranged from 6.8% to 72.1% (Poland, 6.8%; Slovenia, 13.5%; Finland, 15.1%; Spain, 16.0%; Sweden, 28.4%; Italy, 37.8%; Germany, 72.1%).16 In our study, 59% of patients with STEMI received DES in 2009. The differences observed between countries may be related to uncertainty concerning the risk/benefit ratio of DES use and variations in characteristics of cardiac patients in Europe at that time.17 However, concerns over the safety of DES were not substantiated and there was subsequently an increase in DES implantation.18 The representativeness of these studies’ samples and the heterogeneity of the populations involved may have contributed to the observed differences.
Elderly patients are typically not included in randomized trials due to comorbidities and prescription of multiple medications associated with their age, which limits the evidence on the influence of age on stent type selection.19 Increasing age is associated with increased prevalence of atrial fibrillation and consequently chronic oral anticoagulation.20 However, DES as compared to BMS among elderly patients were associated with lower mortality and myocardial infarction risk, without a significant difference in rates of repeat revascularization.21 Drug-eluting stents appear to be safe and effective in the elderly in clinical practice.22 Nevertheless, more studies are needed to validate this data and to verify the possible effects of antiplatelet agents.22 Our study and others reported older age as an independent predictor of the stent type, with a tendency to less use of DES with increasing age.16,23 A higher use of BMS in very young ACS patients may be explained by the angiographic characteristics of the disease, i.e. if these patients were often being treated in large caliber vessels. In this retrospective study, we did not have sufficiently detailed data on angiographic results to be able to test this interpretation, which should be explored in future studies.
Patients with a history of bleeding are typically considered as having the highest risk of rebleeding with anticoagulation and antiplatelet therapy during and after coronary intervention.24 BMS are therefore recommended rather than DES in patients with anemia.12 Compliance with this recommendation was observed in our results, which showed that anemia was an independent predictor of BMS use.
In this study, patients with a history of anticoagulation and patients with atrial fibrillation were pooled due to administration of anticoagulation therapy,24,25 and this has been associated with high risk of bleeding.26 There is a lack of published data on appropriate antithrombotic strategies in patients with anticoagulated atrial fibrillation presenting ACS and undergoing PCI with stent implantation.27 Nevertheless, the guidelines advise the use of the CHADS228 and HAS-BLED29 scores to assess these patients’ bleeding risk. Both the European Society of Cardiology30 and a North American consensus document31 recommend the use of BMS in patients at high risk of bleeding and discourage the use of DES in patients with atrial fibrillation due to the need for dual antiplatelet therapy after stent implantation.32,33 In the present study anticoagulation and/or atrial fibrillation was an independent predictor of BMS use, showing general compliance with these recommendations.
Our study suggests that previous PCI was an independent predictor of DES use, as in the EUROTRANSFER Registry.16 However, this result should be treated with caution, because information on whether the previous PCI was performed in the same vessel was not available. The lack of data regarding the type of stent previously implanted was also a limitation. In addition, it is unknown whether the PCI performed was due to restenosis or in-stent thrombosis.
PCI in the LAD is associated with a higher rate of both restenosis and in-stent thrombosis, and so DES are recommended whenever possible.34,35 However, there is no evidence of benefit in DES use compared to BMS in nonostial proximal lesions of the LAD, and some authors prefer BMS from a cost/benefit perspective in LAD nonostial proximal lesions.36 The LAD as the infarct-related artery was a strong independent predictor of DES use in this study, as also reported in the EUROTRANSFER Registry16 and the EuroPCI Survey.15 Due to the retrospective nature of this study, we did not have detailed angiographic data, and the distribution and anatomical characteristics of lesions could have influenced the choice of stent type.
We also assessed whether in-hospital characteristics had an influence on the choice of stent type, independently of patient characteristics. Patients initially admitted to hospitals without a catheterization laboratory had a lower probability of having a DES implanted. For example, in the case of STEMI diagnosis, late arrival to referral hospitals and the perception of lack of myocardial viability may have led interventionists to preferentially implant BMS in these patients. Furthermore, cases referred to other hospitals for intervention may have had characteristics not considered in this analysis that may have influenced the choice of stent type, such as general condition and comorbidities.
Current guidelines recommend the use of DES in diabetic patients.2 In this study, diabetes was not an independent predictor of the type of stent implanted. In 2009, when the patients in this study were treated, there was evidence of a higher risk of death with DES compared to BMS in diabetic patients, particularly if the duration of dual antiplatelet therapy was <6 months.37
NSTE-ACS patients were more often treated with DES. This difference most likely results from the anatomic characteristics of the vessel and the lesion. No detailed information regarding these characteristics was available in our database, but it would be interesting to explore the putative mechanisms involved in future studies.
In-hospital mortality was significantly lower in patients treated with DES than in those treated with BMS (p<0.001), as has also been documented in other studies.38–41 This difference could be related to the severity of the event, patients implanted with BMS being more likely to have associated comorbidities.
Study limitationsGiven the retrospective nature of this study, the validity of the conclusions relies on the accuracy and completeness of the original documentation. It was assumed that previous coronary heart disease and cardiovascular risk factors did not exist if nothing was reported in the files regarding such issues. To assess the impact of this decision, we compared the prevalence of the characteristics included in this study with data published by the Portuguese Registry of Acute Coronary Syndromes,13 which were similar. Although we had information on the most important variables to address our objectives, it should be acknowledged that it would have been better to have detailed angiographic data and lesion classification. Additionally, this study only reflects practice in the participating hospitals and does not represent overall stent use in Portugal.
ConclusionsIn this analysis, 65% of ACS patients were implanted with a DES, and this type of stent was less frequently implanted in patients diagnosed with STEMI, patients aged over 80 years, in females, in patients with a previous history of stroke, anticoagulation and/or atrial fibrillation, and anemia. Patients who had previously undergone PCI and those with the LAD as the infarct-related artery more frequently had DES implanted than BMS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThis work was supported by Executive Agency for Health and Consumers (2008 13 12 - EURHOBOP).
This work was supported by the Executive Agency for Health and Consumers (2008 13 12 - EURHOBOP) and the authors would like to thank Prof. Jaume Marrugat, coordinator of the project, for the opportunity to participate in this multicenter European study, within the scope of which we obtained the data used for the current report. The authors gratefully acknowledge the collaboration of the hospitals and local researchers who participated in the EURHOBOP study in Portugal: Centro Hospitalar de Vila Nova de Gaia/Espinho (Vasco Gama Ribeiro, Gustavo Pires de Morais), Centro Hospitalar do Porto (Severo Torres, Mário Santos), Centro Hospitalar Cova da Beira (Miguel Castelo Branco), Centro Hospitalar de São João (Paula Dias, Sílvia Marta Oliveira), Hospital de Faro (Ilídio de Jesus, Jorge Mimoso), Hospital Pedro Hispano (Filomena Monteiro), Unidade Hospitalar de Bragança (Domingos Fernandes), Centro Hospitalar do Alto Ave (João Almeida, Filipa Canário Almeida, Francisco Castro Ferreira), Centro Hospitalar de Lisboa Norte (António Nunes Diogo, Maria José Correia), Hospital de Santo André – Leiria (João Morais, Sidarth Pernencar), as well as Ana Bastos, Luísa Conceição and Ricardo Soares, who participated in the data collection.