Twiddler syndrome is a rare cause of pacing failure in pacemakers and implantable cardioverter-defibrillators that should not be neglected.
We describe a case of twiddler syndrome in a patient with a defibrillator.
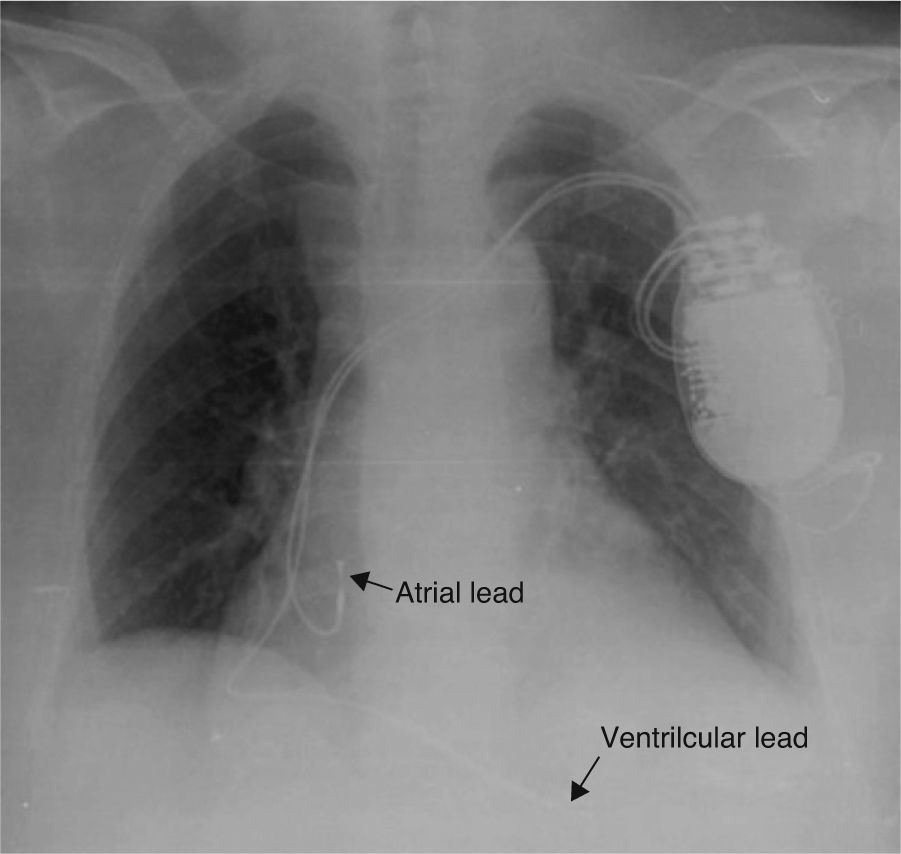
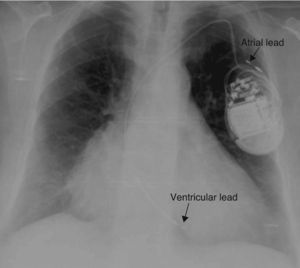
A 74-year-old woman with hypertension, dyslipidemia, and obesity as cardiovascular risk factors was diagnosed with nonobstructive hypertrophic cardiomyopathy with preserved systolic function. During follow-up, she presented sustained syncopal monomorphic ventricular tachycardias and a dual-chamber cardioverter-defibrillator was accordingly implanted; both leads used passive fixation (Figure 1).
At two-month follow-up post-implantation, atrial detection and capture were absent and the ventricular threshold was three times the implant value. Ventricular sensing was preserved. The patient denied conscious manipulation of the device pocket and reported that she had noticed spontaneous rotation of the device. This was corroborated by the family.
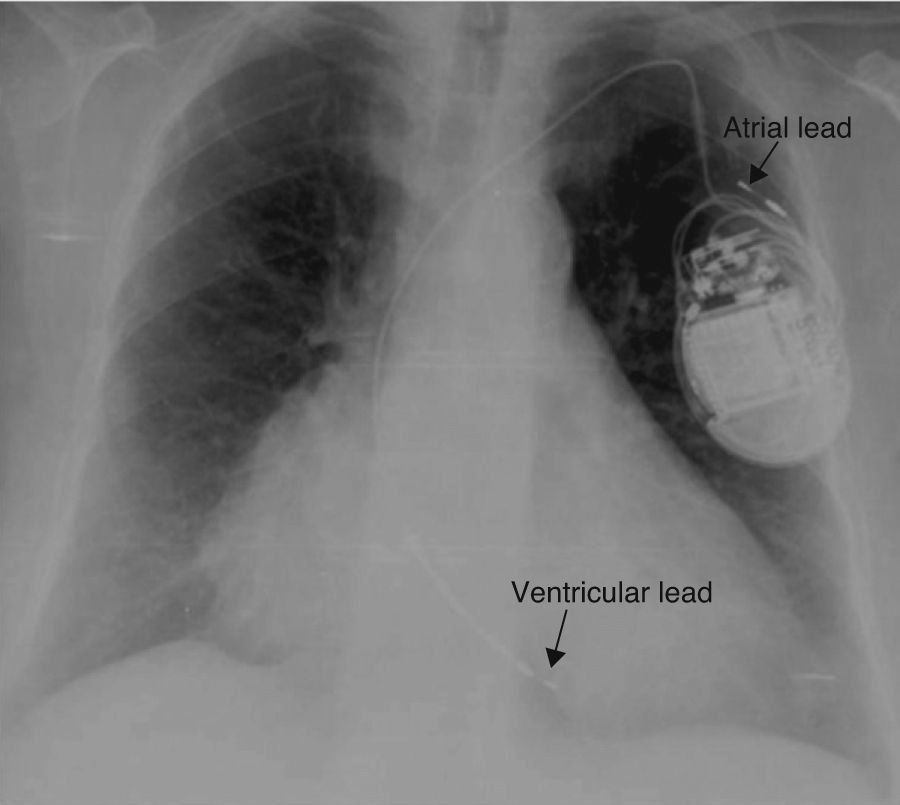
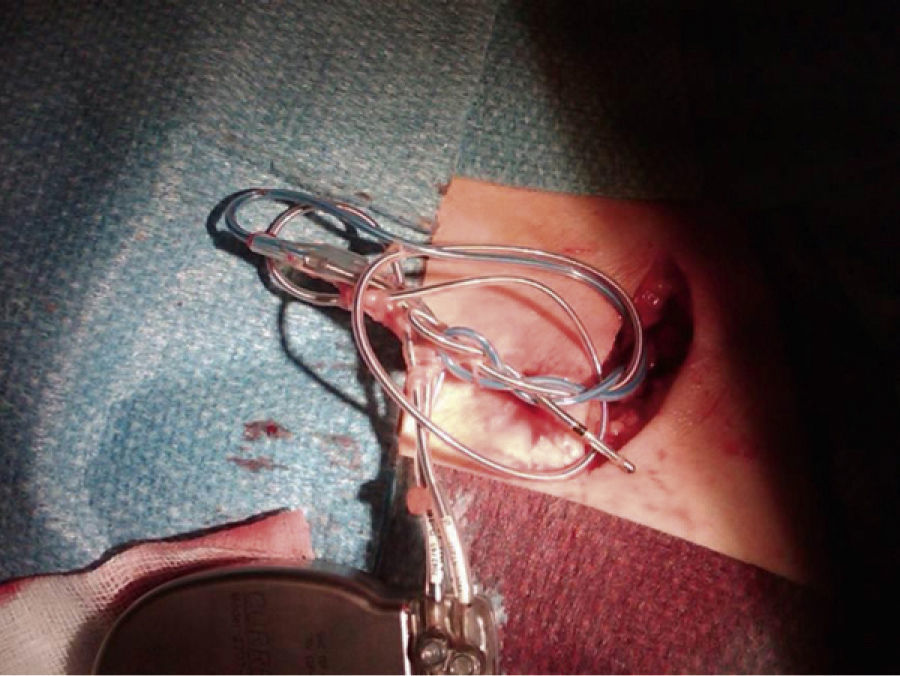
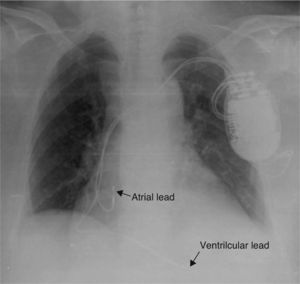
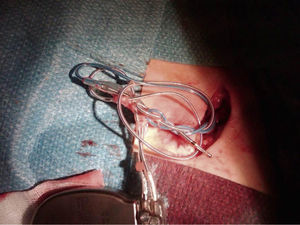
A chest X-ray showed that the generator had rotated and that both leads had become displaced, with the atrial lead situated in the left subclavian vein (Figure 2). Surgery was performed to reposition the ventricular lead and to replace the atrial lead with an active fixation lead (Figure 3); the generator was attached to the pectoral muscle. Subsequent follow-up was unremarkable.
Twiddler syndrome is defined as generator rotation with lead twisting and secondary malfunction of the leads. The causes of this syndrome are not always clear. However, predisposing factors include the creation of a large subcutaneous pocket, obesity, unconscious repetitive movements of the left arm, manipulation of the pocket, or the adoption of repeated poor postural positions. We suggest that the generator be fixed to the muscle plane to prevent the generator from turning and that the patient be given detailed instructions to avoid certain postures or manipulations. The syndrome should be kept in mind, so that device malfunction can be avoided or resolved. In our patient, the atrial lead was mainly affected. In the case of defibrillators, however, inappropriate shock therapies due to incorrect device sensing deserve special attention.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.