Left ventricular pseudoaneurysm is a rare but serious complication of acute myocardial infarction and cardiac surgery. While surgical intervention is the conventional therapeutic option, transcatheter closure can be considered in selected patients with suitable morphology of the pseudoaneurysm. We report a case of successful transcatheter closure of a left ventricular pseudoaneurysm orifice and isolation of the sac using an Amplatzer septal occluder.
O pseudoaneurisma do ventrículo esquerdo é uma complicação rara, mas grave, de enfarte agudo do miocárdio ou pós-cirurgia cardíaca. Enquanto a intervenção cirúrgica é a opção terapêutica convencional, o seu encerramento percutâneo pode ser considerado em pacientes selecionados com morfologia adequada do pseudoaneurisma. Relatamos um caso de oclusão percutânea bem-sucedida do orifício do pseudoaneurisma ventricular esquerdo, isolando o saco através da implantação de um dispositivo de Amplatzer.
Sudden rupture of the myocardial free wall with consequent cardiac tamponade after acute myocardial infarction (MI) is almost always fatal.1,2 When free wall rupture is contained by pericardial adhesions or a new thrombus, preventing exsanguination, the result is a left ventricular (LV) pseudoaneurysm. The true incidence of LV pseudoaneurysm is unknown. In a few reports, however, MI and previous cardiac surgery accounted for 55% and 33%, respectively.3 Mitral valve replacement is a major cause of LV pseudoaneurysm. The location of the pseudoaneurysm differs according to the etiological factor. Post-MI pseudoaneurysm is most commonly located in the inferior or posterolateral wall, while post-surgical pseudoaneurysms are located in the posterior subannular region after mitral valve surgery, the subaortic region after aortic valve surgery, and the right ventricular outflow tract after surgery for congenital heart disease. The incidence of post-MI pseudoaneurysm is declining because of early aggressive treatment of acute ST-elevation MI. Pseudoaneurysm can present with angina, heart failure, arrhythmias, thromboembolism, and even with fatal rupture and tamponade. Untreated pseudoaneurysms can expand and lead to sudden cardiac death due to rupture. In one of the largest series, 48% mortality was reported in untreated cases.4 Although the conventional treatment is surgical intervention, transcatheter device closure is emerging as a new alternative for high-risk surgical candidates.3,5 This was first described by Clift et al. in 2004,6 but experience is still limited to a few reports.
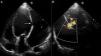
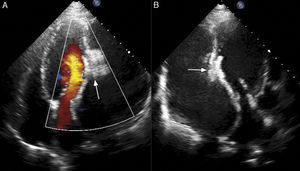
Case reportA 60-year-old male, a chronic smoker for more than 20 years and hypertensive for more than five years, presented to our institute with orthopnea for two weeks, despite optimized treatment for congestive heart failure on an outpatient basis. He had suffered an acute inferior wall MI five months previously, when coronary angiography revealed significant stenosis of the left circumflex artery, and underwent successful angioplasty and stenting. His electrocardiogram showed sinus tachycardia and non-specific persistent ST-T wave changes. Two-dimensional echocardiography and color Doppler (iE33 xMATRIX, Philips Healthcare, Andover, MA, USA) were remarkable, in addition to severe LV dysfunction, for the presence a large pseudoaneurysm lateral to the LV free wall communicating with the ventricular cavity, with the neck measuring 14 mm (Figure 1). The large aperture communicating between the LV and pseudoaneurysm was almost circular, with muscular rims, and the chordal attachment was 12 mm from the nearest margin. In view of the risk of surgery associated with severe LV dysfunction and heart failure, percutaneous closure of the defect was selected by a combined decision of interventionists and surgeons. After written informed consent was obtained in accordance with institutional protocols, retrograde transcatheter closure of the LV pseudoaneurysm was planned using an Amplatzer septal occluder (AGA Medical Corp, Plymouth, MN, USA) under transthoracic echocardiography guidance.
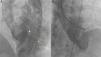
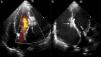
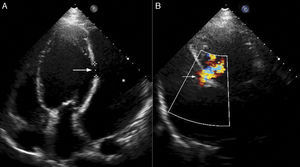
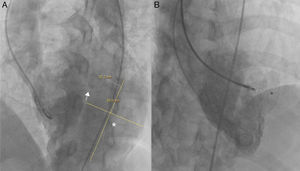
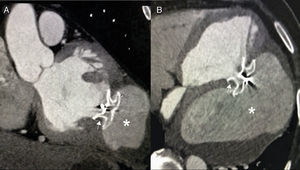
Right axillary artery access was obtained under local anesthesia as the delivery catheter length precluded a femoral artery approach. The LV angiogram with a 6F pigtail (Cordis Corporation, Miami, FL, USA) in left anterior oblique view with cranial angulation showed a large pseudoaneurysm arising from the basal inferolateral LV wall measuring 69 mm×92 mm with a connecting neck measuring 14 mm (Figure 2). A 0.035″ angled-tip hydrophilic glidewire (Terumo Interventional Systems, Japan) was placed in the aneurysm cavity through the communicating orifice using a 6F Judkins Right diagnostic catheter (Cordis Corporation, Miami, FL, USA), then a 0.038″ J-tipped Amplatz Super Stiff exchange wire (Boston Scientific, Marlborough, MA, USA) was substituted for the glidewire with the help of the catheter. A 9F delivery system (Cook Medical, Bloomington, IN, USA) was advanced over the Super Stiff wire and the distal tip was advanced into the pseudoaneurysm cavity. A 20 mm Amplatzer septal occluder, oversized by 6 mm, was deployed across the defect. Post-procedure LV angiography through the same sheath confirmed stable device position with minimal contrast leak foaming through the device. The post-procedure course was uneventful, with improvement in symptoms of heart failure from class III to class II and significantly increased exercise capacity. Follow-up echocardiography after a month showed complete closure of communication between the LV and the pseudoaneurysm (Figure 3). At six-month follow-up computed tomography confirmed a well-placed device, however the pseudoaneurysm was persistent, though not increasing in size and partially thrombosed (Figure 4).
LV pseudoaneurysm is a rare but potentially lethal complication of MI, cardiac surgery, trauma, and infection.7 The largest series, of 290 patients reported by Frances et al., identified MI as the most common cause.3 However, acute inferior wall MI accounted for approximately twice as many cases as anterior wall MI.3 Untreated, LV pseudoaneurysm has a 30%–45% risk of rupture within the first year.8,9 It carries 48% mortality if left untreated.4 Surgical repair of LV pseudoaneurysm also carries a high risk of morbidity and mortality (mortality of 20%–36%).3,5,10
Transthoracic echocardiography is the simplest tool to provide important information on an LV pseudoaneurysm as well as other potential structural problems. However, nonstandard views may be required and the presence of thrombus may lead to underestimation of the size of the cavity. Angiography has been the gold standard for diagnosis, but cardiac magnetic resonance (CMR) is also increasingly used to provide detailed information on location, size, and relationship to adjacent structures and to differentiate true aneurysm from pseudoaneurysm. The use of multimodality imaging, including echocardiography, cardiac catheterization, and/or CMR can provide conclusive morphological information to guide the treatment strategy. While operator experience and volume are important factors, the rarity of this condition and limited experience makes it imperative that each case should be evaluated meticulously if planning for transcatheter intervention.
The first case of nonsurgical treatment of LV free wall rupture used injection of fibrin glue into the pericardium after pericardiocentesis in an elderly woman with cardiac tamponade after acute MI, and had a favorable outcome.11 In the case presented, based on echocardiographic delineation of a 14 mm nearly circular orifice surrounded by a muscular rim, a retrograde transcatheter approach through the axillary access was planned. An axillary artery approach was chosen, as the length of the delivery system was not sufficient for a femoral artery approach. An alternative approach would have been a femoral or jugular venous approach with transseptal puncture. Transseptal access is less suitable for basal inferolateral pseudoaneurysm, in contrast to anterior and apical pseudoaneurysm. Rather, a straighter course with an axillary approach was more suitable for the posterolateral location of the aneurysmal orifice. A transapical approach can be used via percutaneous insertion of the needle into the LV cavity and a delivery system can be placed over the wire across the pseudoaneurysm orifice. Other possible approaches include direct percutaneous puncture of the pseudoaneurysm followed by advancement of the wire through the orifice of the pseudoaneurysm into the LV cavity and snaring it into the aorta and out through the femoral artery to create an arterioventricular rail, or advancing into the left atrium and snaring by a transseptal approach to exteriorize through the femoral vein to create an arterial-venous rail over which the delivery system can be placed.12 However, percutaneous puncture of the pseudoaneurysm was not possible in our patient due to the basal inferolateral location of the pseudoaneurysm.
As there is little guidance in the literature, device selection needs to be individualized depending on the location and size of the pseudoaneurysm, and adjacent structures. Several individual case reports have described successful use of septal occluders, ventricular septal defect occluders, and coils.12–15 The Amplatzer septal occluder was selected for three main reasons: large oversized device >18 mm, adequate rim size of both the retention discs (more than 8 mm), and a relatively small delivery system for the septal occluder. Other choices for devices would have been the Amplatzer Duct Occluder or muscular ventricular septal defect occluder (AGA Medical Corp, Plymouth, MN, USA), which are useful for defects with a long neck.12 In post-MI defects, the margins may be necrotic and friable and may give way during device placement or subsequently. To reduce device instability and the associated risk of device dislodgement, oversize ratios of 1.2–2.0 have been reported for the Amplatzer septal occluder in previous studies.12 We oversized the device by 6 mm (oversize ratio 1.43). The transcatheter approach avoided sternotomy and cardiopulmonary bypass, reducing the significant morbidity associated with surgery. Our patient had a short hospital stay and was completely symptom-free at six-month follow-up.
Transcatheter closure essentially eliminates the symptoms of the pseudoaneurysm by effective and safe device closure of the communication. However, in contrast to surgical repair, persistence of a non-expanding sac with device closure is of concern until long-term results are available. Given the rarity of this condition, even large referral centers have limited experience and outcomes of surgery are also not yet available. Though transcatheter intervention yields promising results without risk, selection of suitable cases for device closure is very important. However, the limited data suggest that percutaneous LV pseudoaneurysm repair may be a good option for patients with high surgical risk.
ConclusionPost-MI pseudoaneurysm is a rare and potentially fatal complication that necessitates early corrective intervention. Retrograde transcatheter device closure of the pseudoaneurysm orifice using a septal occluder is a safe and effective alternative to conventional surgical repair in selected cases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.