Cardiovascular disease (CVD), the leading cause of premature death worldwide, including in Europe,1 is predominantly caused by atherosclerosis, a multifactorial disease strongly associated with dyslipidemia.2 Management of dyslipidemia with statins has resulted in decreases in cardiovascular events and mortality. Unfortunately, although aggressive statin therapy leads to significant reductions in total and low-density lipoprotein (LDL) cholesterol, a proportion of treated patients remain at increased risk for cardiovascular mortality, a phenomenon known as residual cardiovascular risk.3 It thus appears that the benefits of statin therapy have reached a plateau. In addition, many patients are unable to tolerate statins due to adverse effects, particularly muscle-related complications (myalgia, myopathy and rhabdomyolysis) and hepatotoxicity, particularly when under intensive therapy.4 Considering the current unmet needs in the treatment of dyslipidemia, there is considerable potential to reduce CVD mortality and morbidity, which highlights the need for new and effective drugs that can target mediators of atherosclerosis other than cholesterol and lipoproteins.
Oxidative stress is a crucial factor in the development and progression of atherosclerosis, as it is involved in various molecular mechanisms that contribute to vascular damage and foam cell formation.5 Oxidative damage to the endothelium and oxidative modification of LDL contribute significantly to atherogenesis; oxidized LDL activates the endothelium via the production of adhesion molecules, recruiting monocytes and T cells, and thus stimulating immune and inflammatory responses.6 Oxidative stress also appears to be among the multifactorial pathophysiological mechanisms contributing to left ventricular hypertrophy (LVH),7,8 which can be generally viewed as a maladaptive response to chronic pressure overload, and is a major risk factor for atrial fibrillation, diastolic heart failure, systolic heart failure and sudden death in patients with CVD and hypertension.9 Despite the unequivocal role of oxidative stress mechanisms in the progression of CVD, the impact of therapeutic strategies based on antioxidant diets and compounds on cardiovascular risk and events is controversial.10–13
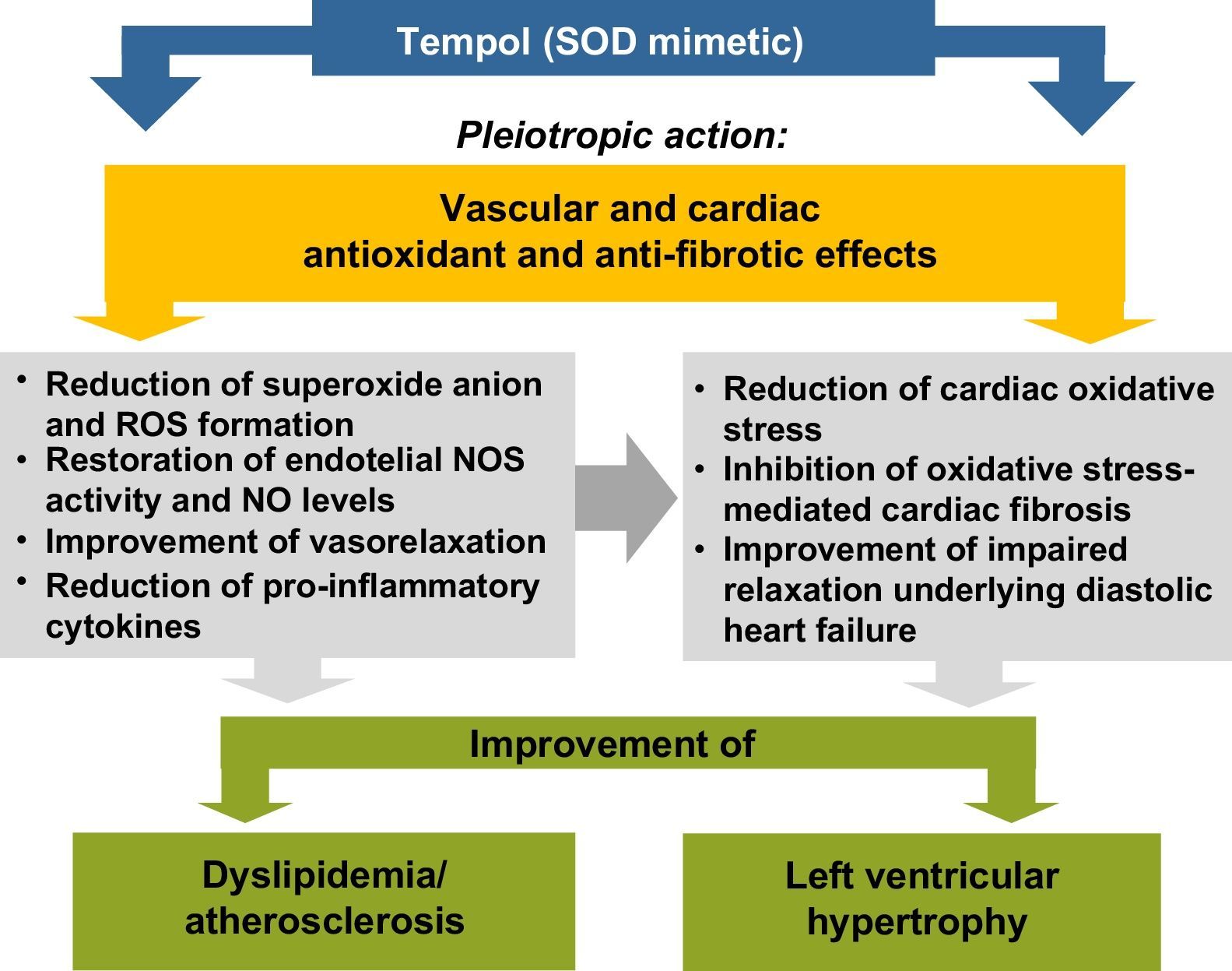
The experimental study by Gonçalves et al.14 in this issue of the Journal assessed the impact of the nitroxide Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl), a superoxide dismutase (SOD) mimetic, on lipid profile and cardiac morphology in the LDL receptor gene knockout (LDLr-/-) mice. After 30 days of a high-fat diet, the animals presented dyslipidemia, with increased concentrations of triglycerides and LDL and very-low density lipoprotein cholesterol, as well as LVH. Tempol (30mg/kg) prevented the development of dyslipidemia and attenuated LVH. The authors claim that Tempol exerted antioxidant effects, thus reducing cardiac oxidative stress, and prevented LVH by alleviating oxidative stress-mediated fibrosis. They also cited previous studies by other groups15–17 suggesting that the beneficial effects of Tempol on dyslipidemia and cardiac damage may be exerted by multiple mechanisms, in pleiotropic-like actions that may explain the restoration of nitric oxide (NO) synthase (NOS) activity and levels of NO, which is recognized as an anti-hypertrophic mediator, as schematized in Figure 1.
Proposed mechanisms to explain the improvement of dyslipidemia and left ventricular hypertrophy by Tempol, a superoxide dismutase mimetic, in agreement with Gonçalves et al.14 NO: nitric oxide; NOS: nitric oxide synthase; ROS: reactive oxygen species; SOD: superoxide dismutase.
However, the study by Gonçalves et al.14 did not present sufficient evidence to confirm this hypothesis. It is also important to assess markers of oxidative stress (such as superoxide anion and peroxynitrite), measures of antioxidant mechanisms (including SOD activity) and NO pathway markers (such as NOS activity and/or NO levels), as well as other molecules, particularly oxidized LDL. Furthermore, determining the impact of Tempol on markers of cardiac oxidative stress and fibrosis, as well as on immune and inflammatory response mediators, will be critical for verifying the suggestion that pleiotropic activity could be responsible for its anti-dyslipidemic and anti-fibrotic cardioprotective action. This question is unanswered by the article, although the effects reported for Tempol and simvastatin (20mg/kg) were similar. Statins exert pleiotropic effects in addition to their cholesterol-lowering properties, but these effects mostly require the use of higher doses, which cannot be confirmed by the present study. Overall, the approach of Gonçalves et al. is an interesting one, targeting oxidative stress to improve dyslipidemia and LVH, but the molecular mechanisms involved need further confirmation in preclinical models before being transposed into clinical practice, in which similar interventions targeting oxidative stress have yet to achieve the expected outcomes in terms of reducing cardiovascular risk and mortality.
Conflicts of interestThe author has no conflicts of interest to declare.
The author acknowledges the support of the Portuguese Foundation for Science and Technology (FCT) and FEDER-COMPETE, through UID/NEU/04539/2013, FCOMP-01-0124-FEDER-028417 and POCI-01-0145-FEDER-007440, as well as Centro 2020 Regional Operational Programmes (CENTRO-01-0145-FEDER-000012: HealthyAging2020).