Red cell distribution width (RDW) is a measure of variation in the size of circulating red blood cells. Recent studies have reported a strong independent relation between elevated RDW and short- and long-term prognosis in various disorders.
The aim of the present study was to investigate the relationship between admission RDW-to-platelet ratio (RPR) and in-hospital and long-term prognosis in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).
MethodsA total of 470 consecutive patients with a diagnosis of STEMI who underwent primary PCI were included in this prospective study. The patients were divided into two groups based on their admission RPR: high (>0.061) RPR group and low (≤0.061) RPR group. The patients were followed for adverse clinical outcomes in-hospital and for up to one year after discharge.
ResultsIn-hospital cardiovascular mortality, major adverse cardiovascular events (MACE), advanced heart failure and cardiogenic shock were significantly higher in the high RPR group (p<0.05). All-cause and cardiovascular mortality, MACE, fatal reinfarction, advanced heart failure, and rehospitalization for cardiac cause were more frequent in the high RPR group in one-year follow-up (p<0.05). High RPR was found to be a significant independent predictor of one-year cardiovascular mortality in multivariate analysis (p=0.003, OR: 3.106, 95% CI: 1.456–6.623).
ConclusionRPR is an inexpensive and readily available biomarker that provides an additional level of risk stratification beyond that provided by conventional risk parameters in predicting long-term MACE and cardiovascular mortality in STEMI.
O índice de dispersão eritrocitária (RDW) é uma medida de variação do tamanho dos glóbulos vermelhos em circulação. Estudos recentes reportaram uma forte relação independente entre o RDW e o prognóstico a curto e a longo prazo em diversas patologias.
O objetivo deste estudo foi investigar a relação entre o rácio do índice de dispersão eritrocitária pelo número de plaquetas (RPR) à admissão e o prognóstico a longo prazo de doentes com enfarte do miocárdio com elevação do segmento ST (STEMI) submetidos a intervenção coronária percutânea (ICP) primária.
MétodosUm total de 470 doentes consecutivos com diagnóstico de STEMI e submetidos a ICP primária foram incluídos neste estudo prospetivo. Os doentes foram divididos em dois grupos baseados no rácio das plaquetas (RPR) na altura do internamento (grupo com RP elevado (> 0,061) e grupo com RPR inferior (≤ 0,061)). Os doentes foram seguidos para registo dos eventos clínicos adversos durante o internamento e um ano após a alta hospitalar.
ResultadosA mortalidade cardiovascular intra-hospitalar, os eventos cardiovasculares adversos major (MACE), a insuficiência cardíaca avançada e o choque cardiogénico foram significativamente superiores no grupo com RPR elevado (p<0,05). A mortalidade por qualquer causa e cardiovascular, os MACE, o reenfarte fatal, a insuficiência cardíaca avançada, o reinternamento por motivos cardíacos foram mais frequentes no grupo com RPR elevado num seguimento a um ano (p<0,05). O RPR elevado foi considerado um fator preditor significativamente independente da análise não multivariada da mortalidade cardiovascular a um ano (p=0,003, OR: 3,106, IC 95%: 1,456-6,623).
ConclusãoO RPR é um biomarcador barato e imediatamente disponível que proporciona um nível de estratificação de risco adicional para além do proporcionado pelos parâmetros do risco convencional na previsão dos MACE a longo prazo e da mortalidade cardiovascular em STEMI.
Despite advances in diagnosis and treatment, ST-segment elevation myocardial infarction (STEMI) remains the most common cause of cardiovascular mortality and morbidity in both developing and developed countries. It is crucial to identify high-risk patients who will require immediate and intensive treatment for myocardial infarction (MI).1,2
Thrombosis and inflammation play an important role not only in the pathophysiology of acute ischemic syndromes,3 but also in the process of atherogenesis, especially in the progression of disease.4 Red cell distribution width (RDW) is a measure of variation in the size of circulating red blood cells and is routinely reported as part of an automated full blood count.5 Recent studies have reported a strong independent relation between elevated RDW and short- and long-term prognosis in various disorders such as coronary artery disease (CAD),6 peripheral vascular disease,7 MI,8 acute and chronic heart failure9,10 and pulmonary embolism,11 as well as in the general population.12 A correlation between RDW and the complexity and severity of CAD has been presented in a previous study.13
Both chronic inflammation and neurohumoral activation lead to an increase in the heterogeneity of circulating erythrocytes, resulting in elevated RDW.14,15
Platelets play a crucial role in the pathogenesis of acute coronary syndrome (ACS). Activated platelets adhere to the vessel wall at the site of ruptured plaque, and initiate arterial thrombus formation, which leads to ischemia or infarction.16,17 Platelets both release markers of inflammation such as soluble CD40 ligand and beta-thromboglobulin18,19 and directly stimulate other cells (including leukocytes, lymphocytes, and monocytes), which can lead to further release of inflammatory markers.20,21 A decreased platelet count was found to be associated with increased infarct size and extent, mean platelet volume and increased platelet aggregation in ACS in previous studies.22–24 There is controversy in the literature concerning the relation between platelet count and short- and long-term mortality in MI.25–28 Although RDW has been related to elevated risk of adverse clinical outcomes in MI, to the best of our knowledge no study has examined the relation between RDW-to-platelet ratio (RPR) and in-hospital and long-term prognosis in patients with STEMI undergoing primary percutaneous coronary intervention (PCI).
The aim of the present study was to investigate the relationship between admission RPR and in-hospital and long-term prognosis in terms of cardiovascular mortality and major adverse cardiovascular events (MACE) in patients with STEMI undergoing primary PCI.
MethodsStudy populationA total of 530 consecutive patients admitted to a high-volume tertiary hospital with a diagnosis of STEMI who underwent primary PCI between September 2009 and November 2010 were included in this prospective study. The inclusion criteria were as follows: (1) presentation within 12 h (18 h for cardiogenic shock) of the onset of symptoms (typical chest pain lasting for >30 min), (2) ST-segment elevation of at least 2 mm in at least two contiguous electrocardiographic (ECG) leads or new onset of complete left bundle branch block, (3) increased serial serum markers of myocardial damage >2-fold over the upper normal range for creatine kinase (CK) and troponin I, (4) treatment by primary PCI (angioplasty and/or stent deployment).
The patients were divided into two groups based on their admission RPR. The high RPR group (n=154) was defined as those with RPR in the third tertile (>0.061), and the low RPR group (n=316) as those with RPR in the lower two tertiles (≤0.061) (p=0.003). All primary PCI procedures were performed in a single high-volume tertiary care center (>3000 PCI/year) by expert interventional cardiologists, who carry out an average of >75 PCI/year and are independent from the study.
The exclusion criteria of the study were as follows: age >80 years, no indication for PCI, coronary anatomy unfavorable for PCI, no follow-up information, clinical evidence of active infection, malignancy, history of anemia and blood transfusions, liver, renal or thyroid disease, history of hematologic disorders, chronic heart failure, acute or chronic active inflammatory disease, history of autoimmune disease, treatment with anti-inflammatory, steroid or chemotherapeutic drugs, and missing or unavailable data on RDW and platelet count. After applying the exclusion criteria, 470 patients were eligible for the study.
Eligible patients were between 18 and 80 years of age and all were able to provide written informed consent, which was a prerequisite for enrollment. The study complies with the Declaration of Helsinki and the trial protocol was approved by the local Ethics Committee.
Analysis of patient dataDemographic information, cardiovascular risk factors and comorbidities (age, gender, smoking habits, family history of CAD, hypertension, hyperlipidemia, history of coronary artery bypass grafting [CABG] surgery, history of PCI and body mass index), reperfusion time and door-to-balloon time, and physical examination data on admission were recorded by systematic review of patient files. The drugs administered to each patient during their hospitalization were also recorded. A 12-lead ECG was recorded in each patient just after hospital admission, and MI type was also obtained.
Transthoracic echocardiography was performed using a system V scanner (GE Vingmed Ultrasound, GE, Horten, Norway) with a 2.5 MHz phased-array probe. Recordings were taken with patients in the left lateral decubitus position. Left ventricular ejection fraction was measured using the modified Simpson's rule.29 Glomerular filtration rate (GFR) for each patient was calculated using the measured plasma creatinine levels and the Modification of Diet in Renal Disease (MDRD) formula for the estimation of renal function.21
Blood collection and assaysOn admission, venous blood was obtained from all patients included in the study before coronary angiography. Platelet, lymphocyte, neutrophil and white blood cell counts were measured as part of the automated complete blood cell count using a Coulter LH 780 Hematology Analyzer (Beckman Coulter Ireland Inc., Mervue, Galway, Ireland). RPR was calculated as the ratio of RDW to platelets, both obtained from the same automated blood sample at admission to the study. Within 24 h of admission, creatine kinase-myocardial band (CK-MB) and troponin I levels were measured for 6 h and the maximum values were recorded. Twelve-hour fasting serum levels of triglycerides and total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol were measured by standard enzymatic methods. Other biochemical measurements were carried out using standard methods.
Coronary angiography and primary percutaneous coronary interventionA chewable 300 mg aspirin tablet and 600 mg loading dose of clopidogrel were given to all patients before coronary angiography. Primary PCI was performed using a percutaneous femoral approach. During the procedure, nonionic low-osmolar contrast media were used and the artery that was presumed to be unobstructed was injected first.
Patients’ angiographic data were evaluated from catheterization laboratory records. Coronary artery stenosis of more than 50% was considered to be clinically significant.
Blood flow in the infarct-related artery (IRA) was graded according to the Thrombolysis in Myocardial Infarction (TIMI) classification.12 Heparin (100 IU/kg) was administered when the coronary anatomy was first defined. After the left and right coronary arteries were visualized, 2.5 μg of nitrate was selectively injected into the IRA to rule out possible coronary spasm.
An angiographic evaluation was made by visual assessment. Primary angioplasty (including balloon angioplasty and/or stent implantation) was performed only for the IRA according to lesion type. For each procedure, procedural success during the acute phase was defined as obstruction and stenosis of the IRA having been reduced to less than 50% stenosis with TIMI II or III flow after primary PCI. After PCI, all patients were transferred to the coronary care unit and given standard therapy for STEMI, which consisted of aspirin, clopidogrel, an angiotensin-converting enzyme inhibitor (ACEI), a beta-blocker, a statin and subcutaneous low molecular weight heparin (enoxaparin). The use of tirofiban was left to the discretion of the operator.
DefinitionsReperfusion time was defined as the time from onset of chest pain to first balloon inflation. Door-to-balloon time was defined as the time between hospital admission and balloon inflation. Patients’ acute clinical status on admission was evaluated according to the Killip classification.23 Acute severe heart failure was defined as New York Heart Association class ≥3. Cardiogenic shock was defined as marked and persistent (>30 min) hypotension with systolic blood pressure of less than 80 mmHg with signs of hypoperfusion due to left ventricular dysfunction, right ventricular infarction, or mechanical complications. Multivessel disease was defined as stenosis of greater than 50% in three major epicardial coronary arteries. Anemia was defined as baseline hemoglobin concentration <13 mg/dl in men and <12 mg/dl in women according to the WHO criteria. Renal failure was defined as GFR estimated by the MDRD equation <60 ml/min/1.73 m2.21 Diabetes was defined as a previous history of the disease, use of dietary treatment, insulin or oral antidiabetic drugs, or fasting venous blood glucose level ≥126 mg/dl on two occasions in previously untreated patients. Hypertension was diagnosed as systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg, or use of antihypertensive drugs. Hyperlipidemia was defined as fasting total serum cholesterol >200 mg/dl, LDL cholesterol >130 mg/dl, or serum triglycerides >180 mg/dl or use of lipid-lowering drugs because of a history of hypercholesterolemia.30
Smoking was defined as the current regular use of cigarettes. Acute stent thrombosis was defined as the abrupt onset of cardiac symptoms (i.e. ACS) along with an increase in the levels of biomarkers or electrocardiographic evidence of myocardial injury within 24 h of stent deployment, accompanied by angiographic evidence of a flow-limiting thrombus near a previously placed stent. Reinfarction was defined based on the guideline for the universal definition of MI.31
Study endpoints and follow-upFollow-up data were obtained from hospital records or by interviewing patients, their families, or their family physicians (directly or by telephone). Major adverse cardiovascular events (MACE) were defined as cardiovascular mortality, reinfarction or repeat target vessel revascularization (TVR) (percutaneous or surgical).
Cardiovascular mortality was defined as unexplained sudden death or death due to acute STEMI, decompensated heart failure or hemodynamically significant arrhythmia. TVR was defined as the need for PCI or CABG surgery due to restenosis or reocclusion of the IRA.
Statistical analysisQuantitative variables were expressed as mean ± standard deviation, and qualitative variables were expressed as a percentage. A comparison of the parametric values between the two groups was performed by a two-tailed Student's t test. Categorical variables were also compared by the likelihood ratio γ2 test or Fisher's exact test. The Spearman correlation coefficient was calculated for the comparison of two data sets. A backward stepwise multivariate Cox regression analysis, which included variables with p<0.1, was performed to identify independent predictors of long-term cardiovascular mortality. Cumulative survival curves for one-year cardiovascular mortality were constructed using the Kaplan–Meier method and compared using the log-rank test. Statistical significance was indicated by a two-sided p value less than 0.05. All statistical analyses were carried out using SPSS statistical software, version 19.0 (SPSS Inc., Chicago, IL, USA).
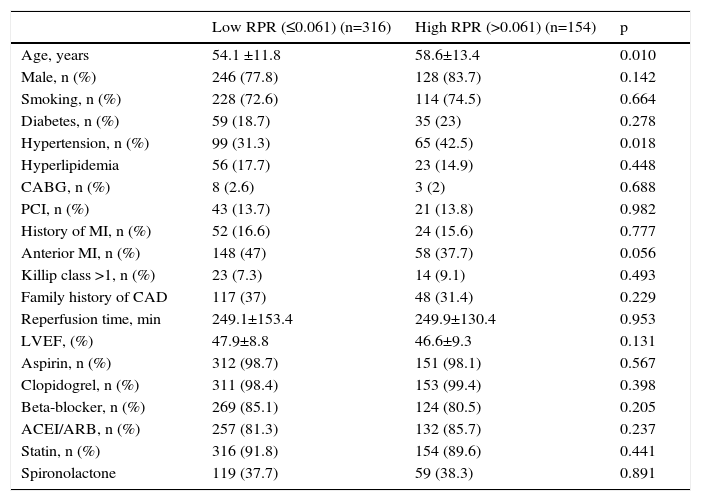
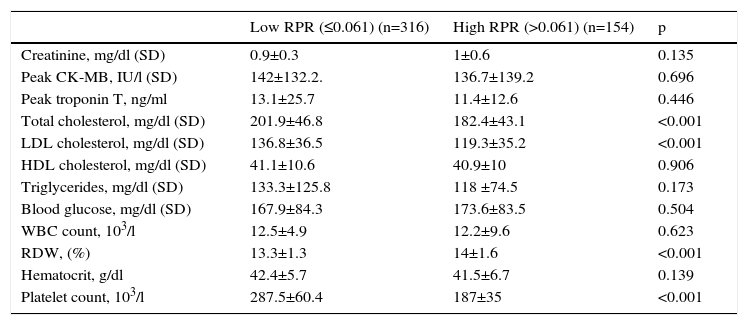
ResultsWe studied 470 of 530 patients who presented with STEMI and underwent primary PCI during the study period. Of these 470 patients (374 men, 96 women), 316 were in the low RPR group and 154 were in the high RPR group. Baseline demographic and clinical characteristics of the patients according to RPR groups are summarized in Table 1. The mean RPR of the study population was 0.057 (range: 0.021–0.195). The high RPR group were older (p=0.01) and tended to have a higher frequency of hypertension (p=0.018) than the low RPR group. Male gender, family history of CAD, diabetes, hyperlipidemia, smoking, previous history of CABG, PCI or MI, anterior MI, Killip class, reperfusion time and door-to-balloon time and ejection fraction were not statistically different between the two groups. Patients’ laboratory characteristics on admission according to RPR group are presented in Table 2. Total and LDL cholesterol levels were found to be significantly lower in the high RPR group than in the low RPR group (p<0.001 for both). Basal creatinine, hematocrit, peak CK-MB, peak troponin, HDL cholesterol, triglyceride, blood glucose, and white blood cell count were not statistically different between the groups. As expected, the high RPR group had significantly higher RDW and lower platelet count (p<0.001 for both).
Baseline demographic and clinical characteristics of low and high RPR groups.
| Low RPR (≤0.061) (n=316) | High RPR (>0.061) (n=154) | p | |
|---|---|---|---|
| Age, years | 54.1 ±11.8 | 58.6±13.4 | 0.010 |
| Male, n (%) | 246 (77.8) | 128 (83.7) | 0.142 |
| Smoking, n (%) | 228 (72.6) | 114 (74.5) | 0.664 |
| Diabetes, n (%) | 59 (18.7) | 35 (23) | 0.278 |
| Hypertension, n (%) | 99 (31.3) | 65 (42.5) | 0.018 |
| Hyperlipidemia | 56 (17.7) | 23 (14.9) | 0.448 |
| CABG, n (%) | 8 (2.6) | 3 (2) | 0.688 |
| PCI, n (%) | 43 (13.7) | 21 (13.8) | 0.982 |
| History of MI, n (%) | 52 (16.6) | 24 (15.6) | 0.777 |
| Anterior MI, n (%) | 148 (47) | 58 (37.7) | 0.056 |
| Killip class >1, n (%) | 23 (7.3) | 14 (9.1) | 0.493 |
| Family history of CAD | 117 (37) | 48 (31.4) | 0.229 |
| Reperfusion time, min | 249.1±153.4 | 249.9±130.4 | 0.953 |
| LVEF, (%) | 47.9±8.8 | 46.6±9.3 | 0.131 |
| Aspirin, n (%) | 312 (98.7) | 151 (98.1) | 0.567 |
| Clopidogrel, n (%) | 311 (98.4) | 153 (99.4) | 0.398 |
| Beta-blocker, n (%) | 269 (85.1) | 124 (80.5) | 0.205 |
| ACEI/ARB, n (%) | 257 (81.3) | 132 (85.7) | 0.237 |
| Statin, n (%) | 316 (91.8) | 154 (89.6) | 0.441 |
| Spironolactone | 119 (37.7) | 59 (38.3) | 0.891 |
CABG: coronary artery bypass graft surgery; CAD: coronary artery disease; DM: diabetes mellitus; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; RDW/Plt: red cell distribution width to platelet ratio.
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables respectively.
Baseline laboratory characteristics of low and high RPR groups.
| Low RPR (≤0.061) (n=316) | High RPR (>0.061) (n=154) | p | |
|---|---|---|---|
| Creatinine, mg/dl (SD) | 0.9±0.3 | 1±0.6 | 0.135 |
| Peak CK-MB, IU/l (SD) | 142±132.2. | 136.7±139.2 | 0.696 |
| Peak troponin T, ng/ml | 13.1±25.7 | 11.4±12.6 | 0.446 |
| Total cholesterol, mg/dl (SD) | 201.9±46.8 | 182.4±43.1 | <0.001 |
| LDL cholesterol, mg/dl (SD) | 136.8±36.5 | 119.3±35.2 | <0.001 |
| HDL cholesterol, mg/dl (SD) | 41.1±10.6 | 40.9±10 | 0.906 |
| Triglycerides, mg/dl (SD) | 133.3±125.8 | 118 ±74.5 | 0.173 |
| Blood glucose, mg/dl (SD) | 167.9±84.3 | 173.6±83.5 | 0.504 |
| WBC count, 103/l | 12.5±4.9 | 12.2±9.6 | 0.623 |
| RDW, (%) | 13.3±1.3 | 14±1.6 | <0.001 |
| Hematocrit, g/dl | 42.4±5.7 | 41.5±6.7 | 0.139 |
| Platelet count, 103/l | 287.5±60.4 | 187±35 | <0.001 |
CK-MB: creatinine kinase MB; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; RDW: red cell distribution width; RPR: red cell distribution width to platelet ratio; WBC: white blood cell.
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables respectively.
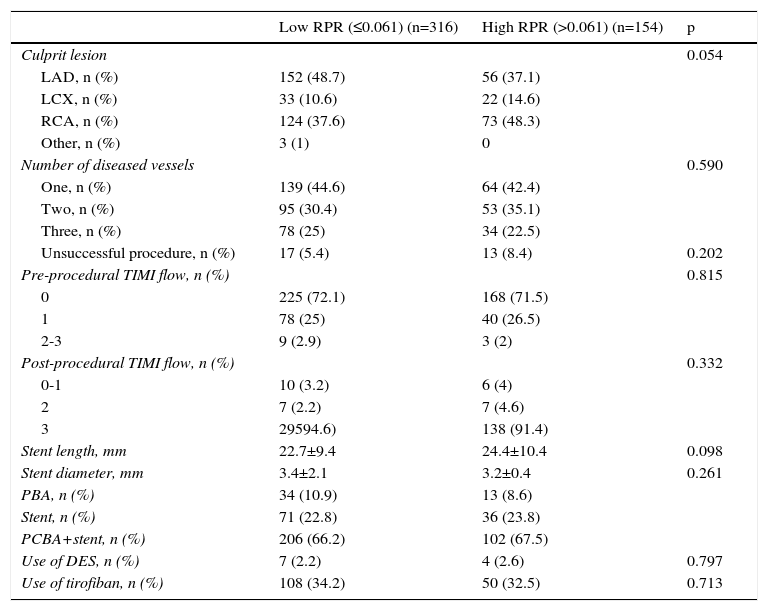
The comparison of angiographic and procedural characteristics between the high and low RPR groups is reported in Table 3. There was no difference between the groups in terms of culprit lesion, number of diseased vessels, procedural success rate, pre-procedural TIMI flow, post-procedural TIMI flow, stent length, stent diameter, primary balloon angioplasty (PBA), stent implantation, PCBA and stent implantation, or use of drug-eluting stents (DES) (all p>0.05). At the time of hospital discharge, the duration of dual antiplatelet therapy after PCI and the use of other cardiac medications including ACEIs, beta-blockers and statins were similar between the groups.
The comparison of angiographic and procedural characteristics between two groups.
| Low RPR (≤0.061) (n=316) | High RPR (>0.061) (n=154) | p | |
|---|---|---|---|
| Culprit lesion | 0.054 | ||
| LAD, n (%) | 152 (48.7) | 56 (37.1) | |
| LCX, n (%) | 33 (10.6) | 22 (14.6) | |
| RCA, n (%) | 124 (37.6) | 73 (48.3) | |
| Other, n (%) | 3 (1) | 0 | |
| Number of diseased vessels | 0.590 | ||
| One, n (%) | 139 (44.6) | 64 (42.4) | |
| Two, n (%) | 95 (30.4) | 53 (35.1) | |
| Three, n (%) | 78 (25) | 34 (22.5) | |
| Unsuccessful procedure, n (%) | 17 (5.4) | 13 (8.4) | 0.202 |
| Pre-procedural TIMI flow, n (%) | 0.815 | ||
| 0 | 225 (72.1) | 168 (71.5) | |
| 1 | 78 (25) | 40 (26.5) | |
| 2-3 | 9 (2.9) | 3 (2) | |
| Post-procedural TIMI flow, n (%) | 0.332 | ||
| 0-1 | 10 (3.2) | 6 (4) | |
| 2 | 7 (2.2) | 7 (4.6) | |
| 3 | 29594.6) | 138 (91.4) | |
| Stent length, mm | 22.7±9.4 | 24.4±10.4 | 0.098 |
| Stent diameter, mm | 3.4±2.1 | 3.2±0.4 | 0.261 |
| PBA, n (%) | 34 (10.9) | 13 (8.6) | |
| Stent, n (%) | 71 (22.8) | 36 (23.8) | |
| PCBA+stent, n (%) | 206 (66.2) | 102 (67.5) | |
| Use of DES, n (%) | 7 (2.2) | 4 (2.6) | 0.797 |
| Use of tirofiban, n (%) | 108 (34.2) | 50 (32.5) | 0.713 |
ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; DES: drug eluting stent; LCX: left circumflex artery; LDA: left descending artery; PCBA: primary coronary balloon angioplasty; RCA: right coronary artery; TIMI: thrombolysis in myocardial infarction.
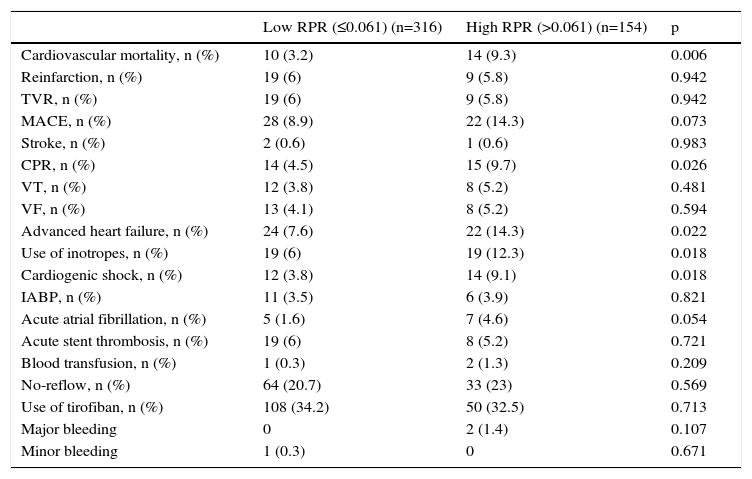
The comparison between high and low RPR groups in terms of in-hospital adverse outcomes after PCI is presented in Table 4. The rate of in-hospital cardiovascular mortality in patients with high RPR was nearly three times that of those with low RPR (9.3 vs. 3.2%, p<0.006). The MACE rate was significantly higher in patients with high RPR than in those with low RPR (14.3 vs. 8.9%, p=0.007). Furthermore, cardiopulmonary resuscitation (p=0.026), advanced heart failure (p=0.022), cardiogenic shock (p=0.018) and inotrope use (p=0.018) were more frequent in the high RPR group.
The comparison of the in-hospital major adverse clinical events between low and high RPR groups.
| Low RPR (≤0.061) (n=316) | High RPR (>0.061) (n=154) | p | |
|---|---|---|---|
| Cardiovascular mortality, n (%) | 10 (3.2) | 14 (9.3) | 0.006 |
| Reinfarction, n (%) | 19 (6) | 9 (5.8) | 0.942 |
| TVR, n (%) | 19 (6) | 9 (5.8) | 0.942 |
| MACE, n (%) | 28 (8.9) | 22 (14.3) | 0.073 |
| Stroke, n (%) | 2 (0.6) | 1 (0.6) | 0.983 |
| CPR, n (%) | 14 (4.5) | 15 (9.7) | 0.026 |
| VT, n (%) | 12 (3.8) | 8 (5.2) | 0.481 |
| VF, n (%) | 13 (4.1) | 8 (5.2) | 0.594 |
| Advanced heart failure, n (%) | 24 (7.6) | 22 (14.3) | 0.022 |
| Use of inotropes, n (%) | 19 (6) | 19 (12.3) | 0.018 |
| Cardiogenic shock, n (%) | 12 (3.8) | 14 (9.1) | 0.018 |
| IABP, n (%) | 11 (3.5) | 6 (3.9) | 0.821 |
| Acute atrial fibrillation, n (%) | 5 (1.6) | 7 (4.6) | 0.054 |
| Acute stent thrombosis, n (%) | 19 (6) | 8 (5.2) | 0.721 |
| Blood transfusion, n (%) | 1 (0.3) | 2 (1.3) | 0.209 |
| No-reflow, n (%) | 64 (20.7) | 33 (23) | 0.569 |
| Use of tirofiban, n (%) | 108 (34.2) | 50 (32.5) | 0.713 |
| Major bleeding | 0 | 2 (1.4) | 0.107 |
| Minor bleeding | 1 (0.3) | 0 | 0.671 |
IABP: intra-aortic balloon pump; MACE: major adverse cardiovascular events (cardiovascular mortality, reinfarction, target-vessel revascularization); TVR: target-vessel revascularization; VF: ventricular fibrillation; VT: ventricular tachycardia.
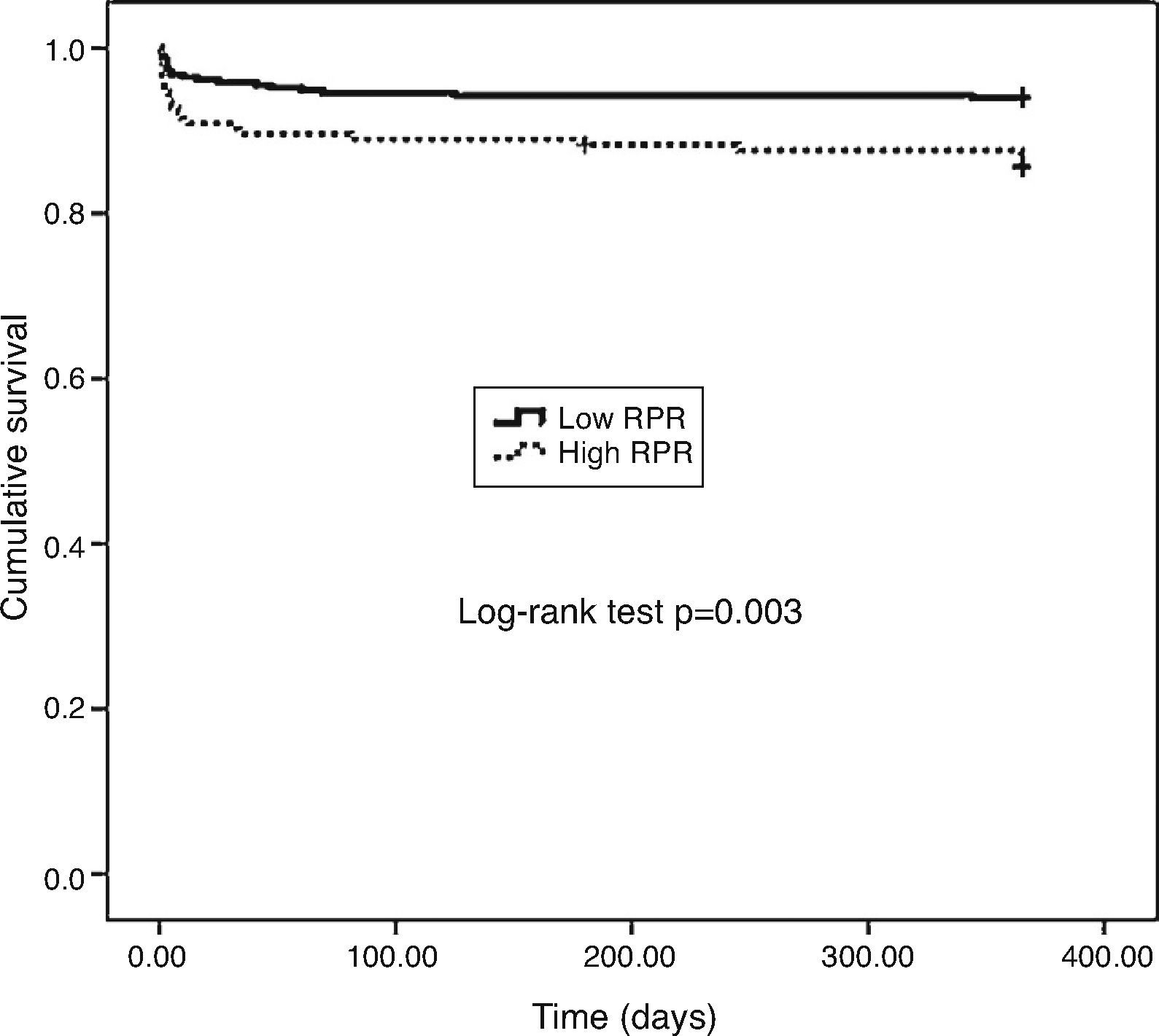
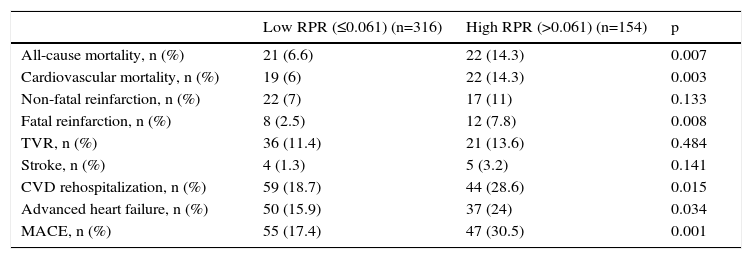
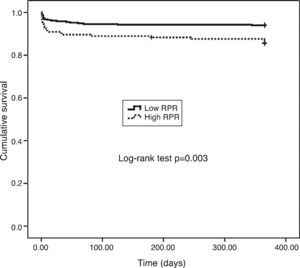
One-year MACE are reported in Table 5. All-cause mortality was significantly higher in the high RPR group compared to the low RPR group (p=0.007). During the course of the study (mean follow-up of 12 months), 41 deaths from cardiac causes occurred out of 470 patients (8.7%). The distribution of causes of cardiovascular mortality were as follows: four patients with unexplained sudden death, 15 with myocardial infarction, 14 with decompensated heart failure, and eight with hemodynamically significant arrhythmia. Cardiovascular mortality was significantly higher in the high RPR group (n=22) than in the low RPR group (n=19) (14.3% vs. 6%; p<0.003). The Kaplan–Meier survival plot for one-year cardiovascular mortality in both groups is presented in Figure 1. Also, the high RPR group had a trend toward a higher incidence of MACE (p=0.034), fatal reinfarction (p=0.008), cardiac rehospitalization (p=0.015), and advanced heart failure (p=0.034). Rates of other complications were not statistically different between the groups.
The comparison of the one-year major adverse clinical events between high and low RPR groups.
| Low RPR (≤0.061) (n=316) | High RPR (>0.061) (n=154) | p | |
|---|---|---|---|
| All-cause mortality, n (%) | 21 (6.6) | 22 (14.3) | 0.007 |
| Cardiovascular mortality, n (%) | 19 (6) | 22 (14.3) | 0.003 |
| Non-fatal reinfarction, n (%) | 22 (7) | 17 (11) | 0.133 |
| Fatal reinfarction, n (%) | 8 (2.5) | 12 (7.8) | 0.008 |
| TVR, n (%) | 36 (11.4) | 21 (13.6) | 0.484 |
| Stroke, n (%) | 4 (1.3) | 5 (3.2) | 0.141 |
| CVD rehospitalization, n (%) | 59 (18.7) | 44 (28.6) | 0.015 |
| Advanced heart failure, n (%) | 50 (15.9) | 37 (24) | 0.034 |
| MACE, n (%) | 55 (17.4) | 47 (30.5) | 0.001 |
CVD: cardiovascular disease; MACE: major adverse cardiovascular events (cardiovascular mortality, reinfarction, target vessel revascularization); TVR: target-vessel revascularization.
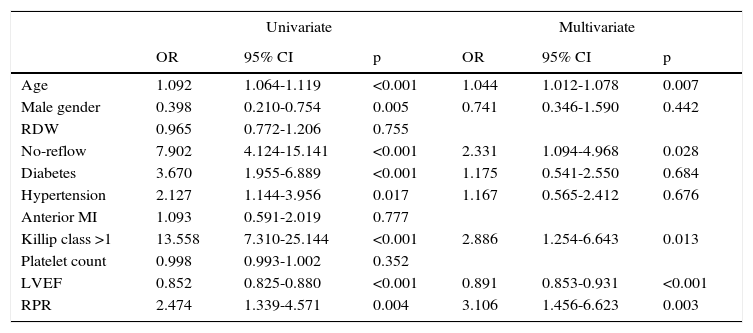
The independent predictors for one-year cardiovascular mortality, including age, male gender, hypertension, RDW, no-reflow, diabetes, hypertension, anterior MI, Killip class >1, platelet count, LVEF, and RPR were included in a Cox proportional hazard model and analyzed in a stepwise fashion. In multivariate analysis, RPR >0.061 (hazard ratio [HR] 3.106, 95% confidence interval [CI] 1.456–6.623, p=0.003), age (HR 1.044, 95% CI 1.012–1.078, p=0.007), no-reflow (HR 2.331, 95% CI 1.094–4.968, p=0.028), Killip class >1 (HR 2.886, 95% CI 1.254–6.643, p=0.013), and LVEF (HR 0.891, 95% CI 0.853–0.931, p<0.001) were significant independent predictors of one-year cardiovascular mortality after adjustment for other risk factors such as diabetes, hypertension, anterior MI and platelet count which had been found to be significant predictors of one-year cardiovascular mortality in univariate analysis (Table 6).
Univariate and multivariate logistic regression analyses for predictors of one-year cardiovascular mortality in STEMI.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.092 | 1.064-1.119 | <0.001 | 1.044 | 1.012-1.078 | 0.007 |
| Male gender | 0.398 | 0.210-0.754 | 0.005 | 0.741 | 0.346-1.590 | 0.442 |
| RDW | 0.965 | 0.772-1.206 | 0.755 | |||
| No-reflow | 7.902 | 4.124-15.141 | <0.001 | 2.331 | 1.094-4.968 | 0.028 |
| Diabetes | 3.670 | 1.955-6.889 | <0.001 | 1.175 | 0.541-2.550 | 0.684 |
| Hypertension | 2.127 | 1.144-3.956 | 0.017 | 1.167 | 0.565-2.412 | 0.676 |
| Anterior MI | 1.093 | 0.591-2.019 | 0.777 | |||
| Killip class >1 | 13.558 | 7.310-25.144 | <0.001 | 2.886 | 1.254-6.643 | 0.013 |
| Platelet count | 0.998 | 0.993-1.002 | 0.352 | |||
| LVEF | 0.852 | 0.825-0.880 | <0.001 | 0.891 | 0.853-0.931 | <0.001 |
| RPR | 2.474 | 1.339-4.571 | 0.004 | 3.106 | 1.456-6.623 | 0.003 |
CI: confidence interval; DM: diabetes mellitus; LVEF: left ventricular ejection fraction; MI: myocardial infarction; OR: odds ratio; RDW: red cell distribution width; RPR: red cell distribution width/platelet.
The main findings of the present study were as follows: (1) the rate of in-hospital cardiovascular mortality was nearly three times higher in the RPR group than in the low RPR group; (2) the rates of in-hospital and one-year MACE were significantly higher in patients with high RPR than in those with low RPR; (3) one-year all-cause mortality was significantly higher in the high RPR group compared to the low RPR group; (4) one-year cardiovascular mortality was significantly higher in the high RPR group; and (5) in multivariate analysis, RPR >0.061, age, no-reflow, Killip class >1, and LVEF were found to be significant independent predictors of one-year cardiovascular mortality after adjustment for other risk factors.
To the best of our knowledge, this is the first study to demonstrate the relationship between RPR and in-hospital and long-term prognosis in terms of cardiovascular mortality and MACE in patients with STEMI undergoing primary PCI.
RDW is a good indicator of variation in the size of circulating erythrocytes and can easily be measured by routine analysis of the complete blood count. Increased RDW reflects the heterogeneity of cell sizes in the peripheral blood smear.32 The most common conditions reported in recent studies in which elevated RDW is seen include ineffective red cell production (due to iron deficiency, vitamin B12 or folate deficiency, hemoglobinopathies, and renal dysfunction), increased red cell destruction (such as hemolysis), blood transfusion, thrombotic thrombocytopenic purpura, inflammatory bowel disease and pregnancy.33–35
The prognostic significance of admission RDW has been reported by Uyarel et al. in patients with non-ST-segment elevation myocardial infarction (NSTEMI) undergoing primary PCI. This study demonstrated that higher baseline RDW was associated with elevated risk of in-hospital and long-term cardiovascular mortality and longer hospital stay.36 RDW was also found to be an important independent predictor of all-cause long-term mortality in NSTEMI.14 Moreover, a graded positive independent relation between admission and discharge RDW values and risk of all-cause mortality and development of new-onset heart failure was found in patients with MI in a study by Dabbah et al.7 A raised baseline RDW was found to be independently associated with abnormal reperfusion and increased risk of in-hospital and long-term cardiovascular mortality in STEMI patients undergoing primary PCI.37 In another study, a significant independent association was demonstrated between RDW and the presence and severity of CAD as assessed by the SYNTAX score.38 The prognostic value of RDW regarding MACE was reported in different large populations with heart failure in previous studies.9 In addition, raised RDW was identified as a strong independent predictor of all-cause mortality in an unselected population of male patients referred for coronary angiography in a study by Cavusoglu et al.39 The relation of RDW with all-cause mortality has been assessed not only in various diseases, but also in healthy subjects. Patel et al. found that for every 1% increment in RDW, the all-cause mortality risk increased by 22%.12 In our study, the in-hospital and long-term prognostic value of RPR was investigated in patients with STEMI undergoing primary PCI. In-hospital cardiovascular mortality, one-year all-cause mortality and one-year cardiovascular mortality were all found to be significantly higher in the high RPR group, in agreement with previous studies. Also, the rates of in-hospital and one-year MACE were higher in the high RPR group, similar to some previous studies.
The reasons for the relationship between RDW and MACE in STEMI are unclear. The main possible mechanisms include inflammatory and neurohormonal activation, which have a crucial role in the initiation and progression of the atherosclerotic process, resulting in CAD and MI.40 Elevated RDW has been reported as a marker of severe inflammation in previous studies.41,42 Lippi et al. showed a relationship between generalized inflammation and increased levels of RDW. They also demonstrated an association between RDW and high-sensitivity C-reactive protein and erythrocyte sedimentation rate, which are reliable indicators of inflammation.43 Inflammation might contribute to increased RDW by inhibiting erythropoietin-induced erythrocyte maturation and impairing iron metabolism.44,45 Furthermore, raised RDW may reflect the production of humoral mediators by bone marrow. In the early phase of acute STEMI, infarction-related inflammatory cytokines and neurohumoral mediators are activated and plasma erythropoietin levels increase. These changes ultimately pave the way for an increase in the heterogeneity of circulating erythrocytes.46,47 Neurohumoral activation may also accelerate erythropoiesis.48
It has been reported that humoral mediators contribute to major adverse clinical events through different mechanisms.49 The renin-angiotensin system, via angiotensin II, may affect erythropoiesis by regulating erythropoietin levels and through direct stimulation of erythroid progenitor cells.48 Furthermore, adrenergic activation may elicit a bone marrow response and increase erythropoietin levels independently of hemoglobin levels in patients with STEMI.47 Increased RDW has been reported to be associated with early signs of subclinical atherosclerosis such as elevated intima-media thickness and incidence of carotid plaque.50 In our study, hypertension was observed more in the high RPR group, which supports the positive relation of increased RDW with severity of inflammation and oxidative stress. However, total and LDL cholesterol were lower in the high RPR group, similar to the results of Uyarel et al.’s study.36
Platelets play an important role in the pathogenesis of ACS. A decreased platelet count was found to be associated with increased infarct size and extent, mean platelet volume and platelet aggregation in MI in previous studies.22–24 Fagher et al. demonstrated that mean platelet count was significantly lower in patients with MI as compared to healthy controls and patients with peripheral arterial disease. The changes in platelet count during MI seemed to parallel the changes in platelet function.22 Also, Pizzulli et al. demonstrated that platelet count was lower in patients with unstable angina pectoris compared to stable CAD after coronary angiography. In this study, patients with unstable angina requiring immediate PCI had an even lower platelet count and higher mean platelet volume than the rest of the population with unstable angina.24 In our study, the high RPR group had significantly lower platelet count compared to the low RPR group. This may be associated with or preceded by a systemic increase in platelet destruction rate in MI that is not completely compensated for by an increase in platelet production rate. Moreover, platelet consumption at the site of the culprit lesion may contribute to thrombus formation in MI and thus to a further increase in platelet destruction. The combination of increased RDW and decreased platelet count, as seen in the high RPR group, predicted in-hospital and long-term prognosis of STEMI treated by primary PCI in our study. Similarly to our study results, platelet count was found to be lower in high burden thrombus formation than in low burden thrombus formation in STEMI patients undergoing primary PCI in a study by Yusuf et al.51
There are conflicting results in the literature in terms of the relation between admission platelet count and cardiovascular mortality and MACE in ACS. Mueller et al. reported an association between low platelet count and cardiovascular mortality in NSTEMI. However, Azab et al. did not demonstrate an independent relation between platelet count and cardiovascular mortality.14,25 In our study, in agreement with previous publications, there was no relation between admission platelet count and in-hospital and long-term prognosis in STEMI patients undergoing primary PCI. RPR was found to be a more powerful predictor of in-hospital and long-term prognosis in STEMI than RDW or platelet count alone. Moreover, RPR was shown to be a significant independent predictor of one-year cardiovascular mortality after adjustment for other risk parameters.
Study limitationsThe present study has some limitations. First, it was performed in a single center and is thus subject to selection bias. Second, we only measured Hb levels, not other factors which affect RDW such as iron, vitamin B12, and folate. Third, even though we speculate that neurohumoral activation may be an important link between increased RDW and adverse outcomes in CAD, we did not measure inflammatory and neurohumoral markers including C-reactive protein, brain natriuretic peptide, other proinflammatory cytokines, norepinephrine, or angiotensin II. Fourth, we could not evaluate changes in RPR in response to aggressive treatment due to lack of serial measurements of RDW and platelet count.
ConclusionWe demonstrate that higher RPR is associated with an increased risk for in-hospital and long-term MACE, all-cause mortality and cardiovascular mortality in STEMI patients undergoing primary PCI. RPR is an inexpensive and readily available biomarker that provides an additional level of risk stratification beyond that provided by conventional risk parameters in predicting long-term MACE and cardiovascular mortality in STEMI patients undergoing primary PCI.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.