Diastolic dysfunction is highly prevalent and a key pathophysiological contributor to several cardiovascular diseases, especially heart failure with preserved ejection fraction. In addition, some evidence suggests diastolic dysfunction is a risk factor for major adverse cardiovascular events. This study aimed to systematically review the evidence and to quantify the association between diastolic dysfunction and risk of cardiovascular events and death.
MethodsMEDLINE was systematically searched from 1974 up to October 2017. We included cohort studies that assessed diastolic function in adults in the community, providing a definition of diastolic dysfunction regarding the occurrence of any cardiovascular event or mortality. For the quantitative analysis, relative risk estimates comparing individuals with versus without diastolic dysfunction were combined using a random effects model.
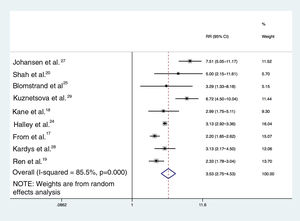
ResultsNineteen studies were identified for inclusion in the systematic review, assessing a total of 63 802 participants. Nine studies were included in the meta-analysis. Diagnostic criteria and classification of diastolic dysfunction differed substantially between studies. The median prevalence of diastolic dysfunction in studies including individuals with and without diastolic dysfunction was 35.1% (range 5.3-65.2%). Comparing diastolic dysfunction with normal diastolic function, the summary relative risk estimate for cardiovascular events or mortality was 3.53 (95% CI: 2.75-4.53; I2=85.5%; nine studies).
ConclusionsAlthough the definitions found in the literature differ, the diagnosis of diastolic dysfunction is associated with a 3.53-fold increased risk of cardiovascular events or death. This finding highlights the importance of developing easily applicable and consensual diagnostic criteria, as well as fostering research on effective treatment strategies when diastolic dysfunction is identified in the subclinical stage.
A disfunção diastólica (DD) é muito prevalente e representa um mecanismo fisiopatológico central para várias doenças cardiovasculares, especialmente para a insuficiência cardíaca com fração de ejeção preservada. Além disso, alguns estudos sugerem que a DD se associa a um aumento do risco de eventos cardiovasculares. Este estudo pretende determinar se a DD é um preditor de eventos cardiovasculares e mortalidade através de uma revisão sistemática e meta-análise.
MétodosFoi realizada uma pesquisa na Medline, desde 1974 até outubro de 2017. Foram incluídos estudos de coorte que avaliassem a função diastólica em adultos da comunidade, comparando participantes com e sem DD, no que diz respeito ao desenvolvimento de eventos cardiovasculares ou morte. Na meta-análise, os riscos relativos foram combinados usando um modelo de random-effects analysis.
ResultadosForam identificados dezanove estudos para a revisão sistemática, avaliando um total de 63 802 participantes, dos quais nove foram incluídos na meta-análise. Observámos que os critérios de diagnóstico e classificação de DD foram bastante diferentes entre os estudos. A prevalência mediana de DD foi 35,1% (variabilidade 5,3%-65,2%). A presença de DD associou-se a um aumento significativo do risco relativo combinado de evento cardiovascular ou mortalidade (3,53; IC 95%: 2,75-4,53; I2=85,5%; 9 estudos).
ConclusõesApesar da heterogeneidade na definição de DD, a sua presença está associada a um aumento marcado do risco de eventos cardiovasculares ou morte. Este resultado realça a importância de desenvolver critérios objetivos e consensuais para o diagnóstico de DD, de modo a promover a sua identificação numa fase subclínica e eventualmente estimular uma investigação dirigida à abordagem terapêutica precoce.
Diastolic dysfunction (DD) is a commonly used term that denotes the presence of pathophysiological changes in cardiac function which include abnormal relaxation, increased myocardial stiffness and increased end-diastolic pressure.1
The prevalence of DD is increasing and is now higher than that of systolic dysfunction.2 According to a recent systematic review, DD affects approximately 36% of the population older than 60 years.3 Echocardiography is often used to assess diastolic function. However, no single echocardiographic parameter is considered sufficiently accurate and reproducible to establish the diagnosis of DD and several parameters must be combined for the diagnosis. Recently, a joint effort involving the European Association of Cardiovascular Imaging and the American Society of Echocardiography set out to harmonize the assessment of diastolic function and to develop a new definition of DD.4
DD is closely associated with several cardiovascular risk factors, including hypertension, obesity and diabetes.5–8 Furthermore, DD is mechanistically involved in the cardiovascular changes that accompany common cardiac diseases, such as stable coronary artery disease, myocardial infarction and cardiomyopathies, with a very strong link to heart failure with preserved ejection fraction (HFpEF).9 DD is one of the best predictors of exercise capacity in patients with heart failure and after myocardial infarction.10 More interestingly, it has also been suggested that increasing severity of DD increases the risk of heart failure1 and is predictive of all-cause mortality.11 However, to the best of our knowledge, the available evidence has not yet been systematically reviewed.
This study aimed to systematically review cohort studies assessing the association between DD and the incidence of major adverse cardiovascular events (MACE) and death, and to quantify the strength of this association using meta-analytical methods.
MethodsData sources and queryThis study was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.12 Studies were identified by searching the PubMed electronic database (MEDLINE) and scanning reference lists of articles. The following search terms were used: (“Diastole”[MeSH] OR diastolic OR “myocardial relaxation” OR “cardiac relaxation” OR “myocardial stiffness” OR “cardiac stiffness”) AND (“Prognosis”[MeSH] OR “Heart Failure”[MeSH] OR “Mortality”[MeSH]) AND (community OR “general population”). A language filter was used to restrict the search to English, Portuguese and Spanish papers. There were no date restrictions applied to the electronic searches – all reports from 1974 until October 26 2017 (when the last search was conducted) were eligible. There were no other methodological filters.
Eligibility criteriaCohort studies that included adults from the community assessing the impact of DD on cardiovascular events (including heart failure, myocardial infarction, and hospitalization from cardiovascular cause) and/or mortality (both cardiac and all-cause) over time were eligible. Studies in specific population groups, such as end-stage renal disease on dialysis, were excluded. Only studies clearly describing how DD was defined and providing data on incident MACE and/or mortality were selected for inclusion. Studies comparing outcomes between individuals with and without DD were eligible for meta-analysis. Studies that only assessed diastolic function parameters as continuous variables, without defining a cut-off to distinguish DD from normal diastolic function, were excluded.
Study selection and data collectionAfter studies were identified using the search query, they were screened by one investigator (M.A.) based on the title and abstract. Eligibility was assessed and article data were extracted by two reviewers independently (M.A., R.L.) using a standardized form. Any disagreement was subsequently resolved by the two authors. Information was extracted from each included report on the following: characteristics of participants (number of individuals, age, gender, ethnicity, risk factors such as hypertension, diabetes, smoking, body mass index, systolic blood pressure, diastolic blood pressure, medication, left ventricular [LV] hypertrophy, systolic dysfunction, heart failure, coronary artery disease) and diastolic echocardiographic measures (e’ velocity, E/e’ ratio, left atrial size, tricuspid regurgitation velocity, E/A ratio); imaging method used for the diagnosis of DD and diagnostic criteria; and type of outcome measure (MACE and/or mortality).
To avoid double counting of a cohort, one set of results was selected when multiple publications were available for the same cohort. Priority was given to the study with the longest follow-up.
Regarding the studies eligible for meta-analysis and not providing the required data in the full-text and supplementary material publications, the first and/or corresponding authors were contacted by email in order to provide the required information.
The methodological quality of all studies was assessed using the revised and validated version of the Methodological Index for Non-Randomized Studies (MINORS),13 as detailed in Supplementary Table 1.
Statistical analysisStudy-specific measures were pooled using random-effects model meta-analysis to provide a single summary estimate. Random-effects model meta-analysis makes allowance for between-study heterogeneity. Pooled estimates along with their 95% confidence intervals (CI) were provided. Heterogeneity between studies was assessed using Q and I2 statistics (I2 values of ≤25%, 50%, and ≥75% represent low, moderate, and high levels of heterogeneity, respectively (www.cochrane-handbook.org).
A forest plot was constructed showing the individual studies with the pooled estimates. Publication bias was assessed using the Egger test and the funnel plot analysis. All statistical analyses were performed using STATA software (version 13.1, StataCorp LP, College Station, TX, USA).
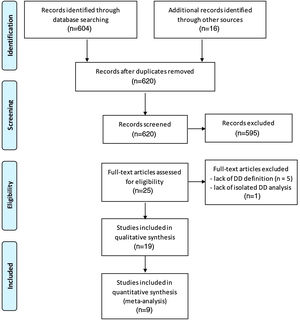
ResultsGeneral characteristics of the included studiesOf a total of 604 initially identified studies, only 12 matched our eligibility criteria and were included (Figure 1). Seven additional studies were included after checking the reference list of the articles, yielding 19 studies for the qualitative analysis. Ten studies were excluded from quantitative analysis (meta-analysis) because they provided data only for patients with DD without a clearly defined group with normal diastolic function (n=4), or provided insufficient data for the required calculations (as full-text and supplementary material publications) and did not respond to multiple contact attempts (n=6).
Flowchart showing search strategy for published data and selection process for inclusion in the systematic review and meta-analysis (according to the PRISMA flow Diagram12).
Nine studies were eventually included in the meta-analysis. For the 19 studies included in the qualitative analysis, the mean follow-up period ranged from one year14 to 11 years.15 Eleven studies were conducted in the USA,11,15–24 six in Europe,14,25–29 one in Israel30 and one in Japan.31 Six of the included studies14,17,21–23,31 were retrospective. Included studies were assessed as high-quality publications (median MINORS score of 17; 25th and 75th percentile of 16 and 18, respectively).
The included studies provided data on 63 802 participants from community-based cohorts, with mean ages ranging from 50.929 to 82 years,14 with some studies focusing on the elderly population.16,20,21,30 Most of the studies had a balanced gender distribution, except for Ren et al.,19 which included 81% males. Kuznetsova et al.29 included only white participants and Brady et al.22 had 57% black participants. As depicted in Table 1, the prevalence of major cardiovascular risk factors was in agreement with what would be expected in cohorts coming from the community. However, Blomstrand et al.25 and From et al.17 focused only on subjects with diabetes. Most of the studies included individuals with systolic dysfunction and only six studies excluded this population.14,19,22–24,31 Kardys et al.28 reported as much as 39% of systolic dysfunction in their baseline population. Symptomatic heart failure was present in eight studies,11,14,18,22,24,25,30,31 and most included individuals with known coronary artery disease (only Aurigemma et al.,16 Tsang et al.,21 Brady et al.22 and Kardys et al.28 used this as an exclusion criterion).
General characteristics of cohort studies assessing the association between diastolic dysfunction and risk of cardiovascular events and/or mortality.
| Study | Cohort (acronym) | n | Patients with LVSD, Yes/No (criteria), percentage | Patients with HF, Yes/No; percentage according to NYHA | Patients with known CAD, Yes/No; percentage | Patients with LVH, percentage; criteria | Age, years (mean/median, SD/IQR) | Male gender, % | Ethnicity, % | Hypertension, % | Diabetes, % | Smokers, % | Mean BMI, kg/m2 | Mean SBP, mmHg | Mean DBP, mmHg | ACEi/ARB, % | Beta-blockers, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Johansenet al.27 | CCHS | 1851 | Yes (LVEF <50%); 0.8% | No | Yes; 16% | 11%; LVMI ≥104 g/m2 (women); ≥116 g/m2 (men) | 57.9 (SD 16.1)a | 43 | N/A | 43a | 10a | NA | 25.4 (SD 3.9)a | 136 (SD 23)a | 79 (SD 12)a | NA | NA |
| Shah et al.20 | ARIC | 5801 | NA | No | NA | NA | 76 (SD 5.1) | 42 | 23% black | 89 | 39 | 6 | 28.8 (SD 5.6) | 131 (SD 18) | 67 (SD 11) | NA | NA |
| Banerjee et al.14 | N/A | 80 | No (LVEF ≤45%) | Yes (100%); NYHA I: 22.5%; II: 43.8%; III: 25%; IV: 8.8% | Yes; 9% | N/A | 82 (SD 8.1) | 34 | N/A | 83 | 24 | N/A | N/A | N/A | N/A | 53 | 44 |
| Blomstrand et al.25 | CARDIPP | 406 | Yes (GLS <-15%); 13% | Yes; 1.5% | Yes; 7.4% | N/A | 60.7 (SD 3.1) | 68 | N/A | 65 | 100 | N/A | 29.8 (SD 4.5) | 136 (SD 15) | 81 (SD 10) | 48 | 35 |
| Kuznetsova et al.29 | FLEMENGHO | 793 | Yes (LVEF ≤50%); 0.8% | N/A | Yes; 3.2% | N/A | 50.9 (SD 15.5) | 49 | 100% white | 41 | 3 | 21 | Women: 26.3 (SD 4.7); men: 26.6 (SD 3.7) | Women: 128 (SD 19); men: 131 (SD 15) | Women: 78 (SD 9); men: 82 (SD 10) | 8 | 15 |
| Di Bello et al.26 | D.A.VE.S | 2142 | Yes (LVEF ≤50%); N/A | No | Yes; 14.8% | 3.5%; LVMI >49.2 g/m27 (men) and >46.2 g/m27 women) | 63 (IQR 56-68) | 54 | N/A | 57 | 14 | 23 | 25 (IQR 23-29) | 140 (IQR 130-150) | 80 (SD 80-90) | 41 | 25 |
| Leibowitz et al.30 | JLCS | 502 | Yes (LVEF <55%); N/A | Yes (11.2%); NYHA I 90.7%; II 7.7%; III 1.4%; IV 0.2% | Yes; 36.8% | N/A | N/A (all individuals >85) | 47 | N/A | 71 | 19 | 3 | 27.2 (SD 4.4) | N/A | N/A | N/A | N/A |
| Vogel et al.23 | RES | 388 | No (LVEF <50%) | No | Yes; 52% | N/A | 67.1 (SD 12.4) | 43 | N/A | 87 | 30 | N/A | 29.2 (SD 6.9) | N/A | N/A | N/A | N/A |
| Halley et al.24 | N/A | 36261 | No (LVEF <55%) | Yes; 3.5% | Yes; 0.6% | N/A | 58.3 (SD 15.4) | 45.6 | N/A | 14.9 | 11.6 | N/A | N/A | 140 (SD 21.6) | 82.1 (SD 11.2) | N/A | N/A |
| Kane et al.18 | OCHFS | 1402 | Yes (LVEF <50%); 2.4%; | Yes; 2.2% | Yes; 16.7% | N/A | 65.2 (SD 9.5) | 49 | >95% white | 42 | 10 | N/A | 28.5 (SD 5.2) | 126 (SD 19.1) | 69.5 (SD 10.4) | 18 | 22 |
| Lam et al.15 | FHS | 1038 | Yes (LVEF ≤45%); 5% | No | Yes; 9% | N/A | 76 (SD 5) | 39 | N/A | 77 | 10 | N/A | 26.6 (SD 4.5) | 147 (SD 22) | N/A | N/A | N/A |
| From et al.17 | N/A | 1760 | N/A | No | Yes; 36% | 33%; LVMI ≥104 g/m2 (women); ≥116 g/m2 (men) | 60 (SD 14) | 49 | N/A | 86 | 100 | N/A | 33 (SD 14) | N/A | N/A | N/A | N/A |
| Kardys et al.28 | Rotterdam Study | 4425 | Yes (“qualitative assessment”); 39% | No | No | N/A | 71.4 (SD 7.3) | 39 | N/A | N/A | 13 | 16 | 27.5 (SD 4.1) | 150 (SD 21) | 80 (SD 11) | 11 | 14 |
| Okura et al.31 | SHFS | 272 | No (LVEF <40%) | Yes; NYHA I 41%; II 58%; IV 1% | Yes; 19% (men), 15% (women) | 61%; LVMI ≥116 g/m2 (men); ≥104 g/m2 (women) | 68.5 (SD 8.7) (men); 69.3 (SD 10.6) (women) | 58 | N/A | 46 | 24 | N/A | N/A | N/A | N/A | 39 | N/A |
| Ren et al.19 | HSS | 693 | No (LVEF <50%) | No | Yes; 100% | 49%; LVMI >90 g/m2 | 65 (SD 10) normal diastole; 72 (SD 9) impaired relaxation; 70 (SD 12) pseudonormal or restrictive | 81 | 59% white, 32% African-American, 13% Asian, 14% other | 71 | 26 | 18 | 28.5 (SD 5.0) normal diastole; 28.2 (SD 4.9) impaired relaxation; 28.6 (SD 5.8) pseudonormal or restrictive | 132 (SD 21) normal diastole; 138 (SD 22) impaired relaxation; 134 (SD 24) pseudonormal or restrictive | 75 (SD 11) normal diastole; 76 (SD 11) impaired relaxation; 71 (SD 11) pseudonormal or restrictive | 46 | 57 |
| Brady et al.22 | N/A | 115 | No (LVEF <50%) | Yes; <0.035% | No | N/A | 58 | 37 | 18% white, 57% black, 22% Hispanic | 71 | 21 | N/A | 31 | N/A | N/A | N/A | N/A |
| Redfield et al.11 | Rochester Epidemiology Project | 2042 | Yes (LVEF ≤50%); 6% | Yes; 2.2% | Yes; 12.2% | N/A | 62.8 (SD 10.6) | 41 | majority white | 25 | 4.5 | 8.9 | 28.4 (5.41) | N/A | N/A | 47.5% LVEF≤40%; 14.2% moderate or severe DD | 22.5% LVEF≤40%; 40.2% moderate or severe DD |
| Tsang et al.21 | N/A | 1160 | Yes (LVEF <50%); 4% | No | No | 3% in group with no events, 6% in group with events; ECG | 75 (SD 7) | 36 | N/A | 48% in group with no events, 63% in group with events | 7% in group with no events, 11% in group with events | N/A | N/A | 142 (SD 23) in group with no events, 146 (SD 23) in group with events | N/A | N/A | N/A |
| Aurigemma et al.16 | CHS | 2671 | Yes (LVEF <45%); 4% | No | No | 7.6% in group with no events, 24.3% in group with events; ratio of observed to expected LV mass/height ratio >1.45 | 72 (SD 5) in group with no events, 74 (SD 6) in group with events | 36% in group with no events, 45% in group with events | 94% white (in group with no events) 94% white (in group with events) | 28% in group with no events, 47% in group with events | 5% in group with no events, 12% in group with events | 12% in group with no events, 12% in group with events | 26 (SD 4) in group with no events, 27 (SD 5) in group with events | 134 (SD 21) in group with no events, 145 (SD 22) in group with events | 70 (SD 11) in group with no events, 73 (SD 13) in group with events | N/A | N/A |
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; CAD: coronary artery disease; DBP: diastolic blood pressure; DD: diastolic dysfunction; ECG: electrocardiography; GLS: global longitudinal strain; HF: heart failure; IQR: interquartile range; LV: left ventricular; LVEF: left ventricular ejection fraction; LVH: left ventricular hypertrophy; LVMI: left ventricular mass index; LVSD: left ventricular systolic dysfunction; N/A: not available; NYHA: New York Heart Association functional class; SBP: systolic blood pressure; SD: standard deviation.
All studies used echocardiography (pulsed-wave Doppler and/or tissue Doppler imaging) to assess diastole and to define DD, except Brady et al.,22 who used cardiac catheterization and measurement of LV pressure. The prevalence of DD in the studies ranged from 5.3%20 to 65.2%,24 with a median prevalence of 35.1%.
Significant heterogeneity was observed regarding the definition and grading of DD. Ten studies11,15,16,18,19,21,23,24,28,29 used a one-level classification tree (criteria were presented for each grade and DD was defined as fulfillment of the criteria for any of these grades), two studies26,27 used a two-level classification tree (criteria for DD were defined and, if fulfilled, subsequent grading took place with additional variables), and seven studies14,17,20,22,25,30,31 only defined the criteria for DD, without grading. As shown in Table 2, there was also significant variability in the core echocardiographic parameters for diagnosis of DD. The most used variable was E/A ratio (10 studies11,15,16,18,19,21,23,27–29), followed by E/e’ ratio (eight studies11,17,20,23,25,27,29,30), E-wave deceleration time (seven studies11,15,18,21,23,28,31), left atrial size (five studies15,20,21,27,29) and pulmonary vein flow indices (five studies11,19,23,29,31), e’ velocity (two studies20,27) and LV end-diastolic pressure (one study22).
Method of assessment of diastolic dysfunction and magnitude of the association with incident cardiovascular events and/or mortality.
| Study | DD imaging technique | DD criteria | DD prevalence, % | Mean lateral e’, cm/s | Mean septal e’e, cm/s | Mean E/e’ ratio (septal or lateral e’) | Mean LA size | TR jet velocity (mean) | Mean E/A ratio | Follow-up (mean) | Primary endpoint | Association measure (HR/RR/OR (95% CI)] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Johansenet al.27 | Echocardiography (including TDI) | e’ <7 cm/s (mean of septal and lateral); grading: E/A >2 and/or LAVi ≥34 ml/m2 and/or E/e’ ≥17 | 52.5 | 7.1 (SD 2.7) (mean of septal and lateral)a | N/A | LAVi 19.1 ml/m2 (SD 6.5) | N/A | 1.11 (SD 0.46)a | 10.9 years | CV death, MI and hospitalization due to HF | HR 2.54 (95% CI 1.61-4) | |
| Shah et al.20 | Echocardiography (including TDI) | According to 2016 guidelines3 | 5.3 | N/A | N/A | N/A | N/A | N/A | N/A | 610 days | All-cause mortality and hospitalization due to HF | Event rate ratio (calculated) 5.36 |
| Banerjee et al.14 | Echocardiography (including TDI) | According to 2007 diastolic heart failure consensus33 | 100 (only patients with DD) | N/A | N/A | 16 (SD 5.74) | N/A | N/A | N/A | 1 year | CV death or hospitalization due to CV event | Incidence 25% |
| Blomstrand et al.25 | Echocardiography (including TDI) | E/e’ ratio >15 | 34.2 | N/A | N/A | 14.4 (SD 4.5) (septal) | N/A | N/A | N/A | 67 (SD 17) months | CV death, myocardial infarction and stroke | HR 3.05 (95% CI 1.18-7.85) |
| Kuznetsova et al.29 | Echocardiography (including TDI) | Impaired relaxation (group 1): ↓ E/A ratio (age-specific) and E/e’ ≤8.5; elevated filling pressure (group 2): normal E/A ratio (age-specific), E/e’ >8.5 or Adur2); combined dysfunction (group 3): ↓ E/A ratio (age-specific) and E/e’ >8.5 | 25.1 | Women: 11.3 (SD 3.6); men: 11.5 (SD 3.8) (mean of septum, lateral, inferior, posterior) | Women: 7.5 (SD 2.4); men: 6.7 (SD 1.9) (mean of septum, lateral, inferior, posterior) | LAVi: women: 21.8 ml/m2 (SD 6.0); men: 24.0 ml/m2 (SD 6.3) | N/A | Women: 1.2 (SD 0.4); men: 1.3 (SD 0.4) | 4.8 years | Cardiac events (MI, coronary revascularization, HF, new-onset angina, cor pulmonale, new-onset AF, life-threatening arrhythmias); CV events (cardiac events plus stroke, TIA, aortic aneurysm, arterial embolism, and peripheral artery revascularization) | HR 1.77 (95% CI: 0.75-4.17) for CV events in group 1 and 2.21 (95% CI: 1.01-3.83) in groups 2+3; HR 2.13 (95%CI: 0.70-6.48) for cardiac events in group 1 and 4.50 (95% CI: 1.73-11.7) in groups 2+3 | |
| Di Bello et al.26 | Echocardiography (including TDI) | According to 2009 guidelines30 | N/A | N/A | N/A | N/A | LA diameter 38 mm (IQR 34-45); LA area 15 cm2 (14-18) | N/A | N/A | 26 (SD 11) months | Cardiac death, MI, CABG or PTCA, stroke, TIA, acute pulmonary edema | OR 1.392 (95% CI 1.313-1.712) |
| Leibowitz et al.30 | Echocardiography (including TDI) | E/e’ >13 | N/A | N/A | N/A | 12.2 (SD 4.9) (mean of septal and lateral tissue velocities) [survivor] | LAVi 36.6 ml/m2 (SD 12.5) [survivor] | N/A | 0.97 (SD1.1) [survivor] | 5 years | All-cause mortality | HR 1.028 (95% CI 0.98-1.084) (E/e’ ratio included as continuous variable in the model) |
| Vogel et al.23 | Echocardiography (including TDI) | Impaired relaxation: grade 1 - E/A ≤0.75, E/e’ <10; grade 1a - E/A ≤0.75, E/e’ >10; pseudonormal pattern: grade 2 - 0.75 | 100 (only patients with DD) | N/A | N/A | 15.5 (SD 5.4) (not specified) | LAVi 41.5 ml/m2 (SD 12.1) | N/A | 1.3 (SD 0.7) | 3.9 years | HF | Cumulative probability of 2.2%, 5.7% and 11.6% at 1, 2 and 3 years, respectively |
| Halley et al.24 | Echocardiography (including TDI) | According to 2002 guidelines34 | 65.2 | N/A | N/A | N/A | N/A | N/A | N/A | 6.2 (SD 2.3) years | All-cause mortality | Mild DD: HR 1.11 (95% CI 0.85-1.47); moderate DD: HR 1.58 (95% CI 1.20-2.08); severe DD: HR 1.84 (95% CI 1.29-2.62) |
| Kane et al.18 | Echocardiography (including TDI) | Mild DD: E/A ratio <0.75; moderate or pseudonormal DD: E/A 0.75 to 1.5, DT >140 ms, plus 2 other Doppler indices of elevated end-diastolic filling pressure; severe DD: E/A ratio >1.5, DT <140 ms, and Doppler indices of elevated LV end-diastolic filling pressure | 39.2 | N/A | 0.08 (SD 0.05) | 10.7 (SD 4.5) (septal) | LAVi 24.7 ml/m2 (SD 8.5) | N/A | N/A | 6.3 (SD 2.3) years | HF | HR 1.81 (95% CI 1.01-3.48) |
| Lam et al.15 | Echocardiography (PW Doppler imaging) | Abnormal relaxation: E/A <0.5, DT >280 ms; restrictive filling: mitral E/A >2.0, DT <120 ms; pseudonormal LV filling: distinguished from normal if LA size ≥sex-specific 80th percentile or LV mass ≥sex-specific 80th percentile or any AF | 36 | N/A | N/A | N/A | N/A | N/A | N/A | 11 years | HF | HR 1.32 (95% CI 1.01-1.71) |
| From et al.17 | Echocardiography (including TDI) | E/e’ ratio >15 | 23 | N/A | N/A | 13 (SD 6) (septal) | LAVi 63 ml/m2 (SD 24) | N/A | N/A | 2.9 (SD 1.8) years | HF | HR 1.61 (95% CI 1.17-2.2) |
| Kardys et al.28 | Echocardiography (PW Doppler imaging) | Impaired relaxation: E/A <0.75 and DT >240 ms; restrictive pattern: E/A >1.50 and DT <150 ms | 11.5 | N/A | N/A | N/A | LA diameter 40 mm (SD 5) | N/A | 0.83 (IQR 0.71-1.00) | 3 years | All-cause mortality | Impaired relaxation: HR 1.55 (95% CI 1.04-2.33); restrictive pattern: HR 7.23 (95% CI 2.16-24.2) |
| Okura et al.31 | Echocardiography (PW Doppler imaging) | LVEF ≥40%+ ≥1 of: (1) DT <140 ms; (2) S/D ratio <1; (3) ARdur-Adur >30 ms | 100 (only patients with DD) | N/A | N/A | N/A | LA diameter 42.9 mm (SD 7.5) males; 40.8 (8.0) females | N/A | 1.3 (SD 0.5) males; 1.2 (SD 0.4) females | 4.4 (SD 1.7) years | All-cause mortality | Incidence rate 6.3% |
| Ren et al.19 | Echocardiography (PW Doppler imaging) | Impaired relaxation: E/A ≤0.75 and systolic dominant pulmonary venous flow; Pseudonormal pattern: E/A=0.75-1.5 and LV diastolic dominant pulmonary venous flow; restrictive pattern: E/A >1.5 and LV diastolic dominant pulmonary venous flow | 52 | N/A | N/A | N/A | N/A | N/A | N/A | 3 years | All-cause mortality, heart disease death, non-fatal MI, hospitalization for HF | Impaired relaxation: HR 0.7 (95% CI 0.4-1.3) for all-cause mortality; HR 1.0 (95% CI 0.3-3.8) for heart disease death; HR 1.7 (95% CI 0.7-4.5) for hospitalization for HF; HR 1.7 (95% CI 0.8-3.7) for non-fatal MI; pseudonormal or restrictive: HR 1.2 (95% CI 0.6-2.4) for all-cause mortality; HR 3.9 (95% CI 1.0-14.8) for heart disease death; HR 6.3 (95% CI 2.4-16.1) for hospitalization for HF; HR 1.3 (95% CI 0.5-3.2) for non-fatal MI |
| Brady et al.22 | Cardiac catheterization and LV pressure measurement | LVEDP ≥15 mmHg and LVEF ≥50% | 100 (only patients with DD) | N/A | N/A | N/A | N/A | N/A | N/A | 63 months | All-cause mortality | Incidence 5% |
| Redfield et al.11 | Echocardiography (including TDI) | Mild DD (impaired relaxation): E/A ≤0.75, ΔE/A <0.5, E/e’ <10, S >D, ARdur | 24.6 | N/A | N/A | N/A | N/A | N/A | N/A | 5 years (longest) | All-cause mortality | Mild DD: HR 8.31 (95% CI 3.00-23.1); moderate or severe DD: HR 10.17 (95% CI 3.28-31.00) |
| Tsang et al.21 | Echocardiography (PW Doppler imaging) | Abnormal relaxation: mitral E/A <0.75 or DT >240 ms; pseudonormal LV filling: mitral E/A=0.75-1.5 and DT 151-240 ms, but LA volume ≥28 ml/m2; restrictive filling: E/A >1.5 or DT ≤150 ms | 60.1 | N/A | N/A | N/A | LAVi 31 ml/m2 (SD 12) in group with no events; 36 ml/m2 (SD 12) in group with events | N/A | N/A | 3.8 (SD 2.7) years | CV death, MI, coronary revascularization, AF, HF, TIA, stroke | HR 1.64 (95% CI 1.1-2.55) |
| Aurigemma et al.16 | Echocardiography (PW Doppler imaging) | E/A <0.7 or >1.5 | N/A | N/A | N/A | N/A | LA diameter 3.8 cm (SD 0.6) in group with no events; 4.0 cm (SD 0.7) in group with events | N/A | 0.95 (SD 0.3) in group with no events; 0.88 (SD 0.4) in group with events | 5.2 years | HF | RR 1.88 (95% CI 1.33-2.68) for E/A ratio <0.7; RR 3.5 (95% CI 1.8-6.8) for E/A >1.5 |
Adur: mitral A-wave flow duration; ARdur: reverse pulmonary vein flow duration; AF: atrial fibrillation; CABG: coronary artery bypass grafting; CI: confidence interval; CV: cardiovascular; DD: diastolic dysfunction; DT: deceleration time; HF: heart failure; HR: hazard ratio; IQR: interquartile range; LA: left atrial; LAVi: left atrial volume index; LV: left ventricle; LVEF: left ventricular ejection fraction; MI: myocardial infarction; N/A: not available; OR: odds ratio; PW: pulsed-wave; PTCA: percutaneous transluminal coronary angioplasty; PV S/D: pulmonary vein systolic forward flow/diastolic forward flow; RR: risk ratio; SD: standard deviation; TDI: tissue-Doppler imaging; TIA: transient ischemic attack; TR: tricuspid regurgitation.
The median incidence of the primary outcome among individuals with normal diastolic function and participants with DD was 3% (range 2.6-16.8%) and 13.1% (range 5.2-37.4%), respectively. Of the 14 studies providing an association measure, 13 showed a significant association between DD and MACE and/or mortality in the multivariate analysis. The strongest association was reported by Redfield et al.,11 with an HR of 8.31 (95% CI 3.00-23.1) for all-cause mortality in individuals with mild DD and an HR of 10.17 (95% CI 3.28-31.00) for patients with moderate or severe DD. Only Leibowitz et al.30 did not find an association (HR 1.028; 95% CI 0.98-1.084) between E/e’ ratio (as a continuous variable) and all-cause mortality.
Two studies found an association between diastolic function variables and cardiovascular events and/or mortality: Shah et al.20 found that abnormal e’, E/e’ ratio, left atrial dimension and left atrial volume index (LAVi) were significantly associated with incident death or heart failure hospitalization, while Vogel et al.23 correlated E/A ratio with incident atrial fibrillation.
Banerjee et al.14 found a significantly worse combined outcome of all-cause mortality and hospitalization in a cohort of patients with DD and increased E/e′ ratio. Okura et al.31 reported that the cumulative survival rate of DD patients, irrespective of a history of heart failure, was significantly lower than in the general population. However, Brady et al.22 showed that the mean mortality in the DD group was similar to the general population, and therefore DD with a normal LVEF, in the absence of coronary artery disease and systolic dysfunction, had an excellent prognosis over a long period (5-6 years). Overall, 17 studies showed that DD was a significant predictor of MACE and/or mortality, while two studies did not find this association.
Based on random-effects model meta-analysis, the pooled estimate for relative risk of MACE/mortality for individuals with DD across nine studies was 3.53 (95% CI 2.75-4.53; I2=85.5%) (Figure 2). Including the studies providing data on hospitalizations and/or mortality (six studies), the pooled estimate using the random-effects model was 3.98 (95% CI 2.91-5.44; I2=84.2%); in addition, including only the two studies providing all-cause mortality as the primary outcome (Halley et al.24 and Kardys et al.28), DD was associated with a 3.13-fold (95% CI 2.92-3.35; I2=0%) increased risk of death.
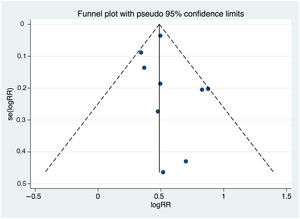
Heterogeneity and publication biasThere were significant differences between individual studies in the magnitude of the association between DD and MACE, as indicated by the statistical test for heterogeneity (Q2=55.0, I2=85.5%, p <0.001). Figure 3 illustrates the funnel plot of studies that excluded small-study effects coming from publication bias (p=0.480 from the Egger test for funnel plot symmetry).
DiscussionThis is the most comprehensive systematic review of cohort studies assessing the association between DD and risk of MACE and death. Overall, most studies (17 studies out of 19) showed DD as a significant predictor of cardiovascular events and death. The quantitative analysis using random-effects model meta-analysis yielded a combined risk of MACE and/or death 3.53-fold higher in the presence of DD; in addition, DD was associated with a 3.13-fold increased risk of death.
Diastolic dysfunction as a predictor of increased risk of cardiovascular events and mortalityResearch during the last decade has shed some light on the pathophysiological changes leading to DD and its deleterious impact on cardiac function. Common risk factors for cardiovascular disease (such as hypertension, obesity, hypercholesterolemia and diabetes) are associated with systemic inflammation, myocardial oxidative stress and coronary microvascular dysfunction, and are significant contributors to myocardial stiffening and LV DD.32 In the cardiovascular risk continuum, intermediate stages of risk such as pre-hypertension6 and non-diabetic metabolic syndrome33 are already associated with deterioration in indices of diastolic function measured by echocardiography and cardiac MRI, which suggests that there is also a continuum of myocardial structural and functional changes that impact on diastole. Indeed, the complex interplay between a systemic low-grade proinflammatory state, endothelial dysfunction and changes in myocardial extracellular space and intrinsic cardiomyocyte properties are now accepted as the new pathophysiological paradigm for HFpEF.34 A previous systematic review and meta-analysis35 showed that asymptomatic LV DD was associated with an increased risk for incident HF (relative risk 1.7; 95% CI: 1.3-2.2), including data from five studies.15–19 Therefore, even subclinical DD seems to be strongly involved in the pathophysiology of HF. That being said, both in patients without symptomatic cardiovascular disease and in patients with full-blown cardiovascular disease (such as coronary artery disease), the presence of DD may indicate a more advanced degree of a specific but complex low-grade inflammatory state and structural and functional myocardial changes that appear to be associated with increased risk of CV events and death.
Causes of heterogeneityIn this meta-analysis significant heterogeneity was observed between studies, which may result from three key factors: different study populations, significant differences in the definition of DD, and different definitions of the primary outcome in each of the studies. All of these are potential limitations of this study and are inherent to the methodological approach adopted, especially regarding the quantitative meta-analysis.
Study populationThis systematic review included different study populations, coming from different countries and continents and including community-derived individuals, elderly populations, and diabetes cohorts. The mean age differed between the study populations, as did gender distribution and ethnicity. As these were community-derived cohorts, the prevalence of cardiovascular risk factors, such as hypertension, diabetes, smoking and obesity, and cardiac diseases (especially coronary artery disease and heart failure) differed significantly between cohorts. This variability in study populations certainly explains a part of the heterogeneity observed in this meta-analysis. On the other hand, it is interesting to observe that despite the variability in the types of individuals included in this analysis, almost all studies showed a consistent association between DD and increased risk of cardiovascular events.
Diastolic dysfunction criteriaGiven the complexity of the pathophysiology of DD, no single echocardiographic parameter can be used to quantify diastole.36 Therefore, over the last two decades, various parameters and classifications of DD have been used in different studies and in different guidelines, which may also explain some of the heterogeneity observed in this study.
As detailed in Table 2, in our systematic review we critically appraised the DD criteria used in different studies and observed striking differences between studies, even though there are published guidelines on diagnosis and grading. For example, the study by Di Bello et al.26 cited the 2009 American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) guidelines,37 but never clarified which variables were used for DD diagnosis. Even Shah et al.,20 who cited the 2016 ASE/EACVI guidelines,4 did not proceed exactly as suggested because they used LA diameter >4 cm to define LA dilatation (the guidelines use indexed volumes) and did not include tricuspid regurgitation jet velocity >2.8 m/s, which is one of the four main parameters in the 2016 guidelines. Moreover, in some studies it was difficult to code diastolic function because of the vagueness of definitions and classifications of DD.
Selmeryd et al.38 examined how the 2009 ASE/EACVI guidelines on the classification of DD were interpreted in the medical community and how variations in the definition of DD affected the reported prevalence. They found that these guidelines have been interpreted differently across studies that cite them with respect to the variables and logical operators used, and that these differences had a substantial impact on the prevalence of DD (range 12-84%).
On the other hand, the 2016 EACVI/ASE consensus on diastolic function4 was intended to simplify the approach to DD classification. It proposes that four variables with high specificity for myocardial disease should be assessed when determining whether LV diastolic function is normal or abnormal, in order to decrease false positive diagnoses of DD: e’ velocity, E/e’ ratio, LAVi and peak tricuspid regurgitation (TR) jet velocity. LV DD is present if three or four parameters are abnormal and inconclusive if only two variables are abnormal. A comparison of the impact of the 2016 ASE/EACVI guidelines on the prevalence and grades of DD in comparison with the 2009 ASE/EACVI guidelines showed that the concordance between the classifications is poor and that the former result in a much lower prevalence of DD, apparently only diagnosing the most advanced cases, leaving many patients diagnosed as having indeterminate diastolic function. One possible explanation might be the inclusion of TR jet velocity, which reflects more advanced and severe DD, resulting in lower sensitivity and higher specificity.39 None of the studies included in this meta-analysis assessed DD using the exact criteria of the 2016 guidelines.
In summary, we strongly believe that it is important to clarify the definition of DD, to correctly assess diastolic function, and to persist in the search for new therapeutic options for DD.
Outcome definitionsIn order to assess the prognosis of DD, we included studies that used diverse outcome definitions, such as different combinations of cardiovascular events, HF hospitalization, the combined endpoint of HF hospitalization and mortality, all-cause mortality or cardiac deaths. Since different outcomes were being assessed, some more comprehensive than others, the strength of the associations will inevitably be different.
Clinical relevanceDespite the heterogeneity between the studies included in this work as discussed above, our findings are significant for daily clinical practice and should drive a shift towards a rapid but careful assessment of diastolic function in most patients, as DD was found to be a consistent predictor of cardiovascular events and death. Therefore, we favor the inclusion of a statement concerning diastolic function in all echocardiography reports, when feasible. Notwithstanding, a universal definition of DD is still lacking and therefore most echocardiography laboratories should adopt the one they feel most confident with and use it for consistency and reproducibility.
ConclusionsIn this systematic review we found a consistent association between DD and the risk of cardiovascular events and death in community-based populations with different risk factors and prevalence of cardiac diseases. Individuals with DD showed a 3.53-fold higher risk of cardiac events or death and a 3.13-fold increased risk of mortality. A simple and widely used definition of DD is urgently needed, not only for user-friendly clinical application but also for the development of new therapeutic trials specifically targeting DD in the subclinical phase.
Conflicts of interestThe authors have no conflicts of interest to declare.