Heart failure is associated with high costs which are mainly the result of recurrent hospital admissions. New strategies to detect early decompensation and prevent heart failure‐related hospitalizations and reduce total health care costs are needed.
Telemonitoring is a novel tool based on the use of recent communication technologies to monitor simple clinical variables, in order to enable early detection of heart failure decompensation, providing an opportunity to prevent hospitalization.
From conventional telemonitoring to more recent strategies using implantable cardiac devices or implantable hemodynamic monitors, the subject is under active investigation. Despite the beneficial effects reported by meta‐analyses of small non‐controlled studies, major randomized controlled trials have failed to demonstrate a positive impact of this strategy. Additionally, evidence regarding the value of newer monitoring devices is somewhat contradictory, as some studies show benefits in prognosis which are not confirmed by others.
This paper provides an overview of the existing evidence on telemonitoring in heart failure and a comprehensive state‐of‐the‐art discussion on this topic.
A insuficiência cardíaca acarreta elevados custos, maioritariamente associados a internamentos recorrentes. Urge encontrar estratégias que possibilitem a deteção precoce dos episódios de descompensação da insuficiência cardíaca, de forma a prevenir as hospitalizações e, assim, reduzir o custo sanitário inerente à doença.
A telemonitorização é uma ferramenta inovadora, baseada na utilização de tecnologias de comunicação recentes capazes de monitorizar variáveis clínicas simples que possibilitem a identificação precoce da descompensação da insuficiência cardíaca, proporcionando a oportunidade de evitar a hospitalização.
Desde a telemonitorização convencional até estratégias mais recentes utilizando dispositivos cardíacos ou monitores hemodinâmicos implantáveis, esta é uma temática sob investigação ativa. Apesar de metanálises prévias de pequenos estudos não controlados terem documentado o potencial benefício da telemonitorização, os principais ensaios clínicos aleatorizados não conseguiram demonstrar o impacto positivo dessa estratégia. Adicionalmente, os dados relativos ao valor dos dispositivos de monitorização mais recentes são contraditórios, na medida em que alguns estudos documentam potencial benefício prognóstico enquanto outros não o conseguem confirmar.
Este artigo fornece uma revisão da evidência científica referente à telemonitorização na insuficiência cardíaca, bem como uma discussão compreensiva acerca do tema.
Heart failure (HF) is associated with high mortality and morbidity, readmission rates and costs.1 Costs related to HF account for 1–2% of all healthcare expenditure, mainly the result of recurrent hospital admissions.2–4 Despite recent advances in medical and device therapy, patients with HF still suffer from repeated hospitalizations due to the combination of progression of the disease, poor adherence to diet and medical therapy, occurrence of comorbidities and limited support.5,6 Thus, this clinical entity remains a major medical and epidemiological problem which carries a heavy economic burden.6 New strategies to detect early decompensation and prevent HF‐related hospitalizations and hence reduce health care costs are needed.
Multidisciplinary HF management programs and HF clinics, considered ‘usual care’ in several European countries, have been successful in reducing all‐cause hospitalization rates.7,8 However, because of geographic barriers, socioeconomic constraints and other obstacles, only a relatively small proportion of HF patients have access to such programs. Interventions have therefore evolved to better monitor HF patients at home.9
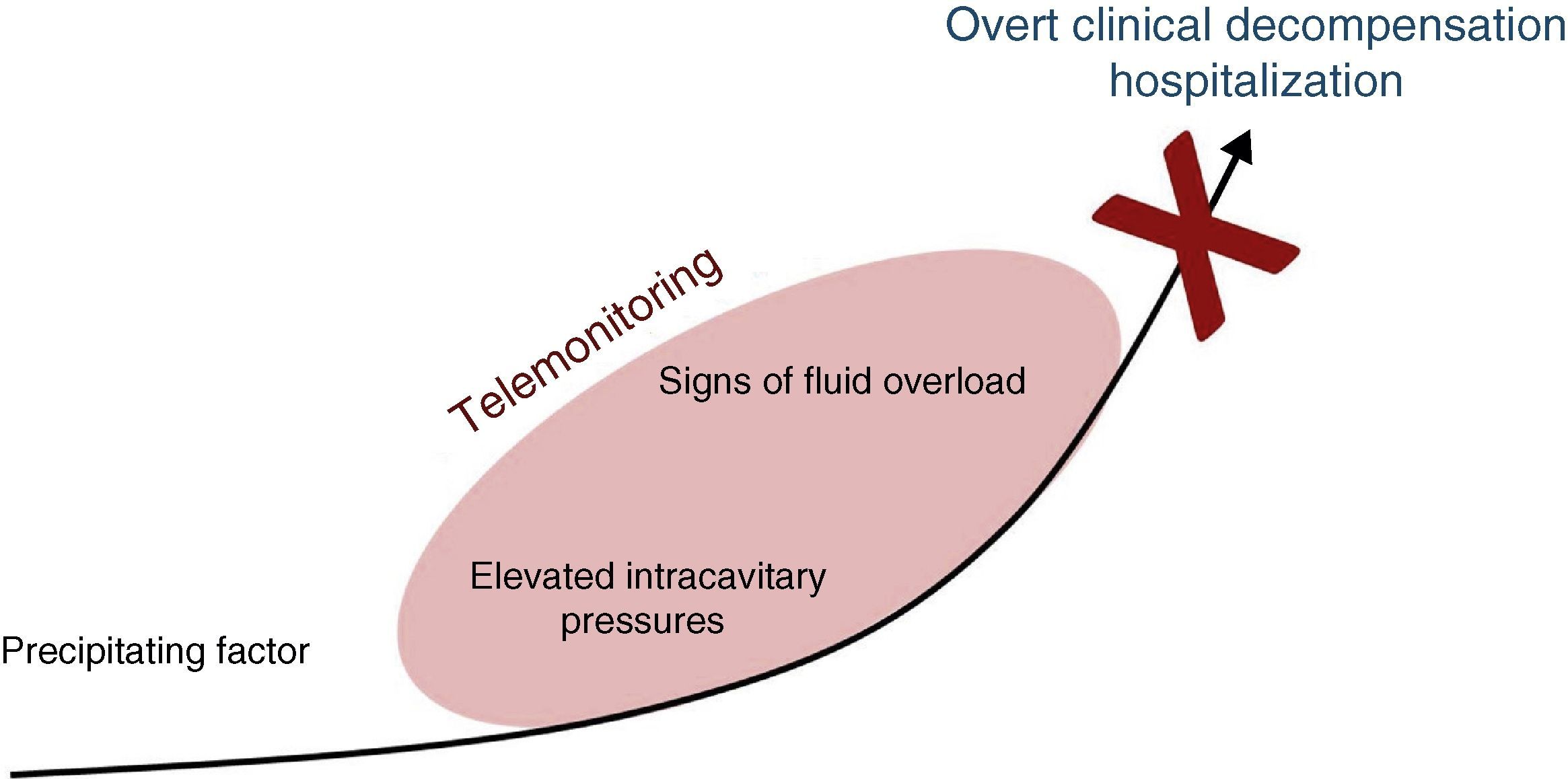
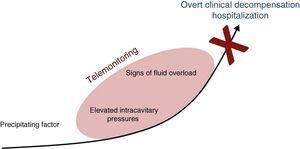
Telemonitoring is a novel tool to improve patient care and adherence which encompasses the use of recent communication technologies to monitor simple clinical variables which are transmitted to the health care provider. Its goal is to detect early signs of heart failure decompensation, providing an opportunity for intervention before the patient requires hospitalization (Figure 1).10–12
Several non‐invasive telemonitoring strategies have been proposed, using regularly scheduled structured telephone interviews or more sophisticated systems, such as electronic transfer of physiological data with remote access control via external, wearable or implantable devices. They have been assessed in retrospective and prospective clinical studies, with conflicting results. This paper sets out to provide a critical review of the current evidence on telemonitoring in HF.
Published meta‐analysesVarious observational studies on HF telemonitoring have called attention to its potential benefit. Two major meta‐analyses aimed to assess the overall effect of HF telemonitoring on prognosis.
In 2009, Klersy et al.13 reviewed 96 articles, comparing multidisciplinary HF approaches by either usual care or remote patient monitoring. The cumulative incidence of events in the usual care approach (in‐person visit) and in remote monitoring strategies (telephone or technology‐assisted monitoring approaches) was compared. A total of 6258 patients were included in randomized controlled trials (RCTs) and 2354 patients in cohort studies, with a median follow‐up of 6 and 12 months, respectively. In RCTs telemonitoring was associated with a significant reduction in mortality compared to usual care (relative risk [RR]: 0.83, p=0.006), total hospitalizations (RR: 0.93, p=0.030) and hospitalizations for HF (RR: 0.71, p=0.001). The combined endpoint of death or first hospitalization showed similar results (RR: 0.86, p=0.001). In cohort studies, telemonitoring was also associated with a significantly lower number of deaths (random‐effects RR: 0.53, p=0.001) and hospitalizations (random‐effects RR: 0.52, p=0.001). Hence, according to this meta‐analysis, remote monitoring significantly reduced the risk of death and hospitalization for any cause in both RCTs and even more markedly in cohort studies.
In 2011, Inglis et al.,14 updating a study by Clark in 2007,15 published an extensive meta‐analysis of RCTs on structured telephone support or telemonitoring compared to standard practice for patients with HF. They included five abstracts and 25 studies, of which 16 evaluated structured telephone support (5613 participants), 11 assessed telemonitoring (2710 participants) and two tested both interventions. Telemonitoring reduced all‐cause mortality (RR: 0.66, p<0.0001) with structured telephone support demonstrating a non‐significant positive effect (RR: 0.88, p=0.08). Both structured telephone support (RR: 0.77, p<0.0001) and telemonitoring (RR: 0.79, p=0.008) reduced HF‐related hospitalizations. In several studies both interventions improved quality of life and reduced healthcare costs. Improvements in therapeutic adherence, patient education and self‐care, and NYHA functional class were also observed. This meta‐analysis thus showed that both structured telephone support and telemonitoring are effective in reducing all‐cause mortality and HF‐related hospitalizations in patients with HF. Additionally, they improve quality of life, reduce costs, and enhance evidence‐based prescription.
Although supporting the use of telemonitoring, the above meta‐analyses present several intrinsic methodological limitations.
The first is inherent to most meta‐analyses and is due to publication bias: only large studies or small studies with positive results, as opposed to those with negative results, tend to be published and therefore included in the analysis.
An additional important limitation refers to the potential lack of quality of the studies included, some of which are small and single‐center.
Moreover, there is also heterogeneity in the studies included regarding selection criteria, design, methodology, telemonitoring technology and follow‐up periods. For example, with regard to the structural telephone support group, the type of professional providing the support and the number of telephone contacts varied. The control groups also varied widely and in some studies the description of this group was too brief or vague to allow replication or comparison with other trials. Additionally, the inclusion and exclusion criteria varied across trials, for example regarding NYHA class or clinical setting (post‐hospital discharge or community settings). This variability leads to a heterogeneous overall population, presenting important limitations to the overall analysis.
Finally, the majority of the included studies were performed in a previous era of HF treatment: recent advances in pharmacological and device therapy, which would now be offered in the standard of care arm, might have changed the overall results; furthermore, the current standard of care of heart failure treatment includes disease management programs and multidisciplinary care, which in previous studies were implemented in the remote monitoring arm only.
Given the above, can it be assumed that the studies included in these meta‐analyses are sufficiently homogeneous to give credibility to the results? And if so, to which patients are the findings applicable? And if telemonitoring and structured telephone support are beneficial, what level of technology is appropriate?
Large RCTs have tried to respond to these questions and their main results will be described in the next section.
Clinical trialsAmong trials conducted in a more contemporary setting, two will be reviewed in view of their size and importance: Tele‐HF and TIM‐HF.
The Telemonitoring to Improve Heart Failure Outcomes (Tele‐HF) trial16 was undertaken to determine the prognostic effect of telephone‐based automated symptom and self‐reported weight monitoring compared with usual care in patients recently hospitalized for heart failure. A total of 1653 patients were enrolled from 33 cardiology centers; 826 were randomly assigned to undergo telemonitoring and 827 to receive usual care. Clinicians were instructed to treat their patients according to guidelines and all patients received educational materials, even in the usual care arm.
The results regarding the primary endpoint of all‐cause readmission or death within 6 months were similar for the telemonitoring and standard‐of‐care groups (52.3% vs. 51.5%, respectively, p=0.75). Secondary endpoint results were also similar for the telemonitoring and standard‐of‐care groups (death: 11.1% vs. 11.4%, respectively, p=0.88; readmission rate: 49.3% vs. 47.4%, p=0.45; readmission for heart failure: 27.5% vs. 27.0%, p=0.81). The time to event for the composite endpoint of readmission or death from any cause was not significantly different between the two groups. Also, there were no significant differences in subgroup analyses, suggesting that no demographic characteristic was likely to predict benefit.
The Telemedical Interventional Monitoring in Heart Failure (TIM‐HF) trial17 was designed to determine whether physician‐led remote telemedical management compared with usual care would result in reduced mortality in HF. A total of 710 optimally treated, stable, ambulatory patients in NYHA class II or III, left ventricular ejection fraction (LVEF) of ≤35% and a history of HF decompensation within the previous two years or with LVEF ≤25% were randomized to usual care (356) or daily telemonitoring (354 patients whose daily electrocardiogram, blood pressure and body weight measurement were sent to telemedical centers with 24‐hour physician availability), with a follow‐up of around two years. There was no difference in the primary endpoint of total mortality (hazard ratio [HR]: 0.97; p=0.87) or in the secondary endpoint composite of cardiovascular mortality or hospitalization due to HF (HR: 0.89; p=0.44). Potential benefit was suggested in the subgroup analysis for those with a prior heart failure hospitalization and LVEF of 25% or higher. Other secondary endpoints included cardiovascular mortality, all‐cause and cause‐specific hospitalizations (all time to first event), days lost due to heart failure hospitalization or cardiovascular death (in % of follow‐up time), and changes in quality of life and NYHA class, with similar results between the arms.
Thus, despite the previous claims of success, in large RCTs a telemonitoring strategy failed to provide benefit over usual care. Although the reported results should prompt a critical reappraisal of telemonitoring, two main questions need to be addressed. Should these findings be considered as definitive evidence against telemanagement for heart failure? Are the assessed features adequate warnings of decompensation?
First, regarding Tele‐HF, it is important to note that adherence was a significant issue, with 14% of those randomized to telemonitoring never using the system and only 55% using it at least three times per week by the end of the study. Furthermore, given the large amount of generated data, physicians’ adherence to the system might also be a concern. Data were transmitted directly to the attending physicians, who were required to document the existence of variances from the basal status and report their responses to these variances. As the authors state, given the lack of systematic recording of these data, it is difficult to assess the extent to which the variances were promptly reviewed and purposeful decisions were made. It can thus be assumed that if monitoring of patients were coupled with more systematic reporting and detailed and prompt interventions, it still might show some benefit. Also, the intervention in this study consisted only of data collection. Newer technologies capable of supporting patient education and self‐care by using daily, real‐time monitoring of physiological data, direct patient feedback and coaching, and a high level of patient–clinician interaction, might achieve positive results.
To summarize, the study's negative findings may be due to low patient and physician adherence to the intervention and/or to inadequate intervention.
In the case of TIM‐HF, the results may have been less influenced by patient compliance (81% of the patients were compliant to the daily transfer of data to telemedical centers) or physician compliance (two telemedical centers provided physician‐led telemedical support 24 hours a day, 7 days a week). However, TIM‐HF presented lack of power to detect clinically relevant differences between the groups, as evidenced by the wide 95% confidence intervals. Given this lack of power, the authors state that the results found do not rule out the potential role of telemonitoring as an addition to the management of HF, but emphasize the need to identify the HF population that could benefit from using this intervention.
Newer monitoring strategiesImplantable hemodynamic monitorsElevations in left ventricular filling pressures and pulmonary artery pressures are closely correlated with clinical congestion, functional limitation, and prognosis in patients with HF. Thus, ambulatory hemodynamic monitoring of these parameters could provide an early warning of potential decompensation as well as facilitate titration of medications on the basis of reliable physiological data.
In this context, the Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS‐HF) trial18 was a multicenter RCT which included 274 NYHA class III or IV HF patients. On top of optimal medical therapy, all patients were implanted with a single transvenous lead in the right ventricular outflow tract to monitor intracardiac pressure and then randomized to two groups: the intracardiac pressure‐guided therapy group (134) and control (140). Even though there were no significant system‐related complications, the use of pressure‐guided therapeutic adjustments failed to reduce total HF‐related events (the therapeutic group presented a non‐significant 21% lower rate of all HF‐related events compared with the control group).
However, these negative results were contradicted by several other studies which documented the potential positive impact of implanted device‐guided monitoring on HF prognosis.
In the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients) trial,19 550 symptomatic HF patients underwent implantation of a wireless pulmonary artery pressure monitoring system and then randomized to daily pulmonary artery pressure‐guided therapy or to usual care. The results were striking: at 6‐month follow‐up the treatment group presented a 28% reduction in hospitalizations (p=0.0002) and a 37% reduction in HF‐related hospitalizations (p<0.0001).
In the HOMEOSTASIS (Hemodynamically Guided Home Self‐Therapy in Severe Heart Failure Patients) trial,20 40 patients with reduced or preserved LVEF, a history of NHYA class III or IV and acute decompensation, were implanted with a left atrial pressure monitor. After an initial period when physicians and patients were blinded to the readings, physician‐directed patient self‐management of left atrial pressure was shown to improve hemodynamics (mean daily left atrial pressure fell from 17.6 to 14.8 mmHg, p=0.003), symptoms (NYHA class decreased by 0.7±0.8, p=0.001), LVEF (7±10%, p<0.001), and outcomes in advanced HF (events tended to be less frequent, HR 0.16, p=0.012).
Implantable cardiac devicesRecent studies have evaluated the ability of implantable cardiac defibrillators and cardiac resynchronization devices to monitor hemodynamic variables. This type of monitoring appears very attractive: additionally to the usual parameters such as percentage of ventricular pacing and the presence of arrhythmias, activity levels, mean heart rates at rest or during exertion and heart rate variability, changes in thoracic impedance and cardiopulmonary filling pressures can also be monitored. This may help in the early detection of acute decompensation.
Increased pulmonary vascular congestion decreases transthoracic impedance, which can be reported by the device before symptom development, giving the possibility of early detection of HF decompensation, for which it could be more sensitive than body weight changes.
This subject is under active investigation but here also, the published results are conflicting.
SENSE‐HF (Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations)21 was a multicenter trial which included 501 HF patients with a newly implanted cardioverter‐defibrillator with or without cardiac resynchronization therapy. The OptiVol algorithm provided intrathoracic impedance measurements but presented low sensitivity and positive predictive value for the detection of HF events in the early period after implantation (six‐month sensitivity and positive predictive value of 20.7% and 4.7%, respectively).
The Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCE‐HF)22 trial included 400 patients with NYHA class II or III symptoms, indication for an implantable cardioverter‐defibrillator (ICD) and a previous HF hospitalization. An ICD with hemodynamic monitoring capability was implanted and patients were randomly assigned to a treatment group in which hemodynamic information was used or a control group (no hemodynamic information available). Mean follow‐up time was 11.6 months. Due to early enrollment termination, the trial was unable to test the primary clinical effectiveness hypothesis adequately. The primary safety endpoint was met, but the rate of HF equivalents was not different between groups.
DOT‐HF (Diagnostic Outcome Trial in Heart Failure)23,24 provided similar results, with 335 patients with chronic HF who had undergone implantation of an OptiVol‐equipped ICD or cardiac resynchronization therapy defibrillator (CRT‐D) were included and randomized to have information available to physicians and patients (access arm) or not (control arm). The primary composite endpoint of all‐cause mortality and heart failure hospitalizations was more common in the access arm (HR 1.52; p=0.063), mainly due to more heart failure hospitalizations (HR 1.79; p=0.022), whereas the number of deaths was comparable (p=0.54). The access arm also presented a higher number of outpatient visits (p=0.0001).
However, in a study by the OptiVol CRT group,25 the device's fluid status alert appeared to improve prognosis by allowing timely detection of HF decompensation and therapeutic intervention. A total of 532 HF patients were included. Acute decreases in intrathoracic impedance were associated with clinical events in 47% of cases and led to drug therapy adjustment in 20% of events. More importantly, the 102 patients in whom the impedance alert was disabled presented a higher rate of combined cardiac death and HF hospitalization (log‐rank test, p=0.007).
Similarly, in the PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) trial,26 monthly review of HF device diagnostic data identified patients at higher risk of HF hospitalization. A total of 694 CRT‐D patients were included. The HF decompensation diagnostic algorithm was based on long atrial fibrillation duration, rapid ventricular rate, high fluid index, low patient activity, high night heart rate or low heart rate variability, and low CRT pacing or ICD shocks. Patients with positive HF diagnostics had an increased risk of HF hospitalization within the next month (HR 5.5, p=0.0001), even after adjustment for other clinical variables (HR 4.8, p=0.0001).
The EVOLVO (Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators) trial27 involved 200 HF patients with ICDs with and without resynchronization therapy and compared remote interrogation with standard patient management (scheduled visits and patients’ response to audible ICD alerts). Reduced healthcare use was shown in the remote monitoring group. Total clinical visits (35% less, p=0.005), visits for heart failure, arrhythmias or ICD‐related events (21%; p=0.001) and time from ICD alert to review (24.8 days in the standard arm vs. 1.4 days in the remote arm, p=0.001) were all reduced. Also, remote ICD monitoring significantly improved quality of life when assessed by the Minnesota Living With Heart Failure Questionnaire (p=0.026).
Clearly, further data are required to validate the use of the fluid index and other device‐based algorithms in HF. Investigation in this area continues with the OptiLink (Optimization of Heart Failure Management using OptiVol Fluid Status Monitoring and CareLink) trial. In this study patients with newly implanted or replaced ICDs with or without resynchronization and with chronic HF in NHYA class II or III and LVEF ≤35% will be randomized to either OptiVol fluid status monitoring through CareAlert notification or regular care (OptiLink ‘on’ vs. ‘off’). The main purpose is to investigate whether early detection of congestion reduces mortality and cardiovascular hospitalization in patients with chronic HF. The study is expected to report initial results in May 2014.
DiscussionDespite notable advances in the understanding of the pathophysiology and treatment of heart failure, it still carries an enormous clinical and economical burden. This is due in great part to hospitalizations.
Detecting decompensation before it leads to hospitalization appears a promising strategy, and so, conceptually, telemonitoring should be a valuable tool to improve outcomes in this population.
In fact, several observational studies and small RCTs have supported this hypothesis. However, the results from major RCTs did not support the benefit of telemonitoring over usual care in HF patients.
Ambulatory hemodynamic monitoring via implanted devices would theoretically offer more accurate and robust data to identify patients at greater risk of decompensation. However, the few published studies still lack consistency in demonstrating their benefit, which must also be weighed against safety concerns due to the invasive nature of the procedure.
Thus, at this point, it is difficult to draw firm conclusions regarding the clinical efficacy of telemanagement (Table 1). Despite the growing interest in telemonitoring in the cardiology community, many questions remain unanswered. Which patients benefit most, and how often? Which parameters should be monitored? How could these parameters be monitored more efficiently? How should responses of health care professionals to the data obtained from monitoring be managed?
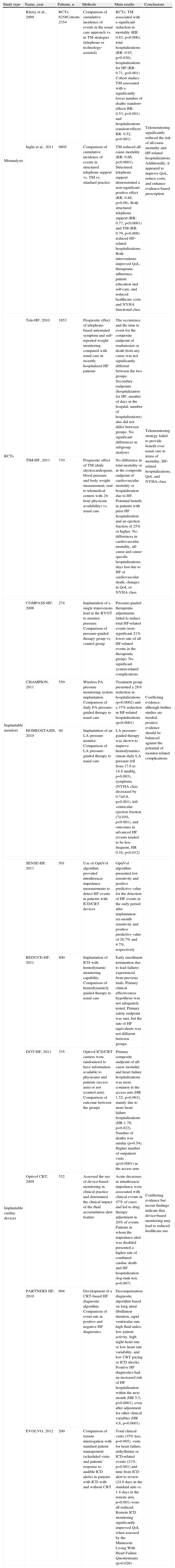
Summary of the major published articles on telemonitoring in HF.
| Study type | Name, year | Patients, n | Methods | Main results | Conclusions |
| Metanalysis | Klersy et al., 2009 | RCTs: 6258Cohorts: 2354 | Comparison of cumulative incidence of events in the usual care approach vs. in TM strategies (telephone or technology‐assisted) | RCTs: TM associated with a significant reduction in mortality (RR: 0.83, p=0.006), total hospitalizations (RR: 0.93, p=0.030), hospitalizations for HF (RR: 0.71, p=0.001) Cohort studies: TM associated with a significantly lower number of deaths (random‐effects RR: 0.53, p=0.001) and hospitalizations (random‐effects RR: 0.52, p=0.001) | Telemonitoring significantly reduced the risk of all‐cause mortality and HF‐related hospitalizations. Additionally, it appeared to improve QoL, reduce costs, and enhance evidence‐based prescription |
| Inglis et al., 2011 | 9805 | Comparison of cumulative incidence of events in structured telephone support vs. TM vs. standard practice | TM reduced all‐cause mortality (RR: 0.66, p<0.0001). Structured telephone support demonstrated a non‐significant positive effect (RR: 0.88, p=0.08). Both structured telephone support (RR: 0.77, p<0.0001) and TM (RR: 0.79, p=0.008) reduced HF‐related hospitalizations. Both interventions improved QoL, therapeutic adherence, patient education and self‐care, and reduced healthcare costs and NYHA functional class | ||
| RCTs | Tele‐HF, 2010 | 1653 | Prognostic effect of telephone‐based automated symptom and self‐reported weight monitoring compared with usual care in recently hospitalized HF patients | The occurrence and the time to event for the composite endpoint of readmission or death from any cause was not significantly different between the two groups. Secondary endpoints (hospitalization for HF, number of days in the hospital, number of hospitalizations) also did not differ between groups. No significant differences in subgroup analyses | Telemonitoring strategy failed to provide benefit over usual care in terms of mortality, HF‐related hospitalizations, QoL and NYHA class |
| TIM‐HF, 2011 | 710 | Prognostic effect of TM (daily electrocardiogram, blood pressure and body weight measurement, sent to telemedical centers with 24‐hour physician availability) vs. usual care | No difference in total mortality or in the composite endpoint of cardiovascular mortality or hospitalization due to HF. Potential benefit in patients with prior HF hospitalization and an ejection fraction of 25% or higher. No differences in cardiovascular mortality, all‐cause and cause‐specific hospitalizations, days lost due to HF or cardiovascular death, changes in QoL or NYHA class | ||
| Implantable monitors | COMPASS‐HF, 2008 | 274 | Implantation of a single transvenous lead in the RVOT to monitor pressure. Comparison of pressure‐guided therapy group vs. control group | Pressure‐guided therapeutic adjustments failed to reduce total HF‐related events (non‐significant 21% lower rate of all HF‐related events in the therapeutic group). No significant system‐related complications. | Conflicting evidence: although further studies are needed, positive evidence should be balanced against the potential of monitor‐related complications |
| CHAMPION, 2011 | 550 | Wireless PA pressure monitoring system implantation. Comparison of daily PA pressure‐guided therapy to usual care | Treatment group presented a 28% reduction in hospitalizations (p=0.0002) and a 37% reduction in HF‐related hospitalizations (p<0.0001) | ||
| HOMEOSTASIS, 2010 | 40 | Implantation of an LA pressure monitor. Comparison of LA pressure‐guided therapy to usual care | LA pressure‐guided therapy was shown to improve hemodynamics (mean daily LA pressure fell from 17.6 to 14.8 mmHg, p=0.003), symptoms (NYHA class decreased by 0.7±0.8, p=0.001), left ventricular ejection fraction (7±10%, p<0.001), and outcomes in advanced HF (events tended to be less frequent, HR 0.16, p=0.012) | ||
| Implantable cardiac devices | SENSE‐HF, 2011 | 501 | Use of OptiVol algorithm provided intrathoracic impedance measurements to detect HF events in patients with ICD/CRT devices | OptiVol algorithm presented low sensitivity and positive predictive value for the detection of HF events in the early period after implantation: six‐month sensitivity and positive predictive value of 20.7% and 4.7%, respectively | Conflicting evidence but recent findings indicate that device‐based monitoring may lead to reduced healthcare use |
| REDUCE‐HF, 2011 | 400 | Implantation of ICD with hemodynamic monitoring capability. Comparison of hemodynamicly guided therapy to usual care | Early enrollment termination due to lead failures experienced from previous trials. Primary clinical effectiveness hypothesis was not adequately tested. Primary safety endpoint was met, but the rate of HF equivalents was not different between groups. | ||
| DOT‐HF, 2011 | 335 | Optivol ICD/CRT carriers were randomized to have information available to physicians and patients (access arm) or not (control arm). Comparison of outcome between the groups | Primary composite endpoint of all‐cause mortality and heart failure hospitalizations was more common in the access arm (HR 1.52; p=0.063), mainly due to more heart failure hospitalizations (HR 1.79; p=0.022). Number of deaths was similar (p=0.54). Higher number of outpatient visits (p=0.0001) in the access arm | ||
| Optivol CRT, 2009 | 532 | Assessed the use of device‐based monitoring in clinical practice and determined the clinical impact of the fluid accumulation alert feature | Acute decreases in intrathoracic impedance were associated with clinical events in 47% of cases and led to drug therapy adjustment in 20% of events. Patients in whom the impedance alert was disabled presented a higher rate of combined cardiac death and HF hospitalization (log‐rank test, p=0.007) | ||
| PARTNERS HF, 2010 | 694 | Development of a CRT‐based HF diagnostic algorithm. Comparison of event rate in positive and negative HF diagnostics | Decompensation diagnostic algorithm based on long atrial fibrillation duration, rapid ventricular rate, high fluid index, low patient activity, high night heart rate or low heart rate variability, and low CRT pacing or ICD shocks. Positive HF diagnostics had an increased risk of HF hospitalization within the next month (HR 5.5, p=0.0001), even after adjustment for other clinical variables (HR 4.8, p=0.0001) | ||
| EVOLVO, 2012 | 200 | Comparison of remote interrogation with standard patient management (scheduled visits and patients’ response to audible ICD alerts) in patients with ICD with and without CRT | Total clinical visits (35% less, p=0.005), visits for heart failure, arrhythmias or ICD‐related events (21%; p=0.001) and time from ICD alert to review (24.8 days in the standard arm vs. 1.4 days in the remote arm, p=0.001) were all reduced. Remote ICD monitoring significantly improved QoL when assessed by the Minnesota Living With Heart Failure Questionnaire (p=0.026) | ||
CRT: cardiac resynchronization therapy; HF: heart failure; ICD: implantable cardioverter‐defibrillator; LA: left atrium; PA: pulmonary artery; QoL: quality of life; RCTs: randomized controlled trials; RVOT: right ventricular outflow tract; TM: telemonitoring.
These are the million‐dollar questions. Future research should focus on these aspects in order to find the most ‘active ingredients’ which can make telemonitoring work.
Perhaps the biggest revolution in this area is the development of tools that analyze the data automatically and provide advice to both patients and health professionals in making care decisions. These can give patients more control in managing their problems and much more personalized health‐care. Mobile phone‐based remote monitoring systems are relatively inexpensive and convenient tools to improve HF home management. Mobile phones are now widely available, inexpensive and portable, enabling patients to be monitored anywhere. Initial studies have shown the potential of this approach in HF home management,28 but further studies are needed.
ConclusionThis review underscores the need for careful assessment of telemonitoring as a disease management system before its widespread adoption. Until the ideal tools are found, dedicated monitoring for HF may be a practical adjunct in selected centers and patients, additional to usual care, but should not replace it as a standard of care so long as the evidence remains conflicting, insufficient and heterogeneous.
Conflicts of interestThe authors have no conflicts of interest to declare.