In 1992, Brugada and Brugada first described a new entity, which became known as Brugada syndrome, that is associated with a high risk of ventricular arrhythmias and sudden cardiac death in patients without structural heart disease. This syndrome is characterized by a distinct electrocardiographic phenotype, type 1 Brugada pattern, consisting of a coved ST-segment elevation (≥0.2 mV) followed by a negative T wave in more than one right precordial lead. This pattern is dynamic, and can be spontaneous or concealed, but is unmasked under certain circumstances, like febrile states.
The authors report a case in which the diagnosis of Brugada syndrome was made in the course of etiologic investigation of recurrent syncope in a febrile state.
Em 1992, Brugada et Brugada descreveram pela primeira vez uma entidade, conhecida atualmente por síndrome de Brugada, associada a aumento do risco de arritmias ventriculares e morte súbita cardíaca em indivíduos sem cardiopatia estrutural. Esta síndrome caracteriza-se por um fenótipo eletrocardiográfico distinto, padrão de Brugada tipo 1, o qual consiste numa elevação (≥0,2mV) «arqueada» do segmento ST seguida de uma onda T negativa em mais de uma derivação precordial direita. Este fenótipo é dinâmico, podendo ser espontâneo ou encontrar-se oculto, sendo desmascarado em múltiplas circunstâncias, nomeadamente em contexto febril.
Os autores apresentam um caso clínico em que o diagnóstico de síndrome de Brugada é efetuado na sequência do estudo etiológico de síncopes recorrentes em contexto febril.
We report the case of a 40-year-old man who went to the emergency department (ED) with fever and productive cough for about two days. While in the ED he had two episodes of syncope preceded by palpitations, sweating and blurred vision, followed by rapid recovery of normal consciousness. He was not under electrocardiographic monitoring during either episode.
The patient reported recurrent syncope in febrile states since childhood, and a few months before this admission had had an episode of pre-syncope preceded by palpitations during the night. He had no other relevant medical history and was not taking any routine medication; on the day of admission he had taken only paracetamol. There was no family history of heart disease, sudden cardiac death (SCD) or recurrent syncope.
On physical examination the patient was normotensive, tachycardic, febrile, and slightly polypneic, with diminished breath sounds bilaterally and no alterations on cardiac auscultation.
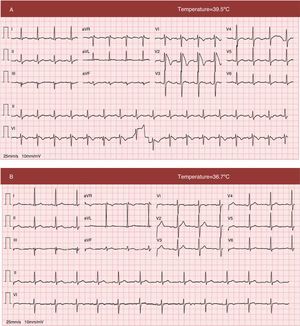
The electrocardiogram (ECG) after the syncopal events (Figure 1A) showed a coved ST-segment elevation in V1 and V2, maximum 0.6 mV in V2, followed by a negative T wave; these alterations in ventricular repolarization are compatible with type 1 Brugada pattern.
12-lead electrocardiogram (A) at admission with fever, showing sinus rhythm at 100 bpm, coved ST-segment elevation in V1 and V2, maximum 0.6 mV in V2, followed by a negative T wave, compatible with type 1 Brugada pattern; QTc is 412 ms; (B) electrocardiogram in apyrexia, showing resolution of the alterations in ventricular repolarization seen in V1 and V2.
Laboratory tests showed elevated inflammatory markers and D-dimers; serum potassium and myocardial necrosis biomarkers were within normal ranges. Following chest computed tomography angiography, which revealed no thromboembolism, the patient was transferred to the intermediate care unit with a diagnosis of community-acquired pneumonia complicated by type 1 respiratory failure. Empirical antibiotic therapy was begun with ceftriaxone and clarithromycin and he was kept under continuous electrocardiographic monitoring.
The patient's clinical course was favorable and his fever resolved within 24 hours. There were no further episodes of syncope or arrhythmia. ECG performed in apyrexia showed resolution of the typical Brugada alterations seen in a febrile state (Figure 1B). Transthoracic echocardiography revealed no structural heart disease.
Since the patient's setting was compatible with Brugada syndrome (BrS), with spontaneous type 1 Brugada pattern and a history of recurrent syncope, he received an implantable cardioverter-defibrillator (ICD) for primary prevention of SCD. He was discharged on the 11th day of hospitalization and advised to monitor and immediately treat any rise in body temperature, and was informed on the need to avoid drugs contraindicated in BrS.
Six months after discharge, the patient was asymptomatic and without arrhythmic events. Genetic screening of his family was underway.
DiscussionBrS is one of the hereditary conditions known as channelopathies, primary cardiac rhythm disturbances caused by ion channel anomalies that result in susceptibility to ventricular arrhythmias, and hence SCD, in individuals without structural heart disease.1,2 It is estimated to be responsible for at least 4% of all sudden deaths and at least 20% of sudden deaths in patients with structurally normal hearts.2
BrS is a hereditary disease with autosomal dominant transmission. In 1998, Chen et al. identified the first mutation associated with the condition, in the SCN5A gene, which codes for the alpha subunit of the cardiac sodium channel.3,4 Mutations in other genes, coding for calcium and potassium channels, were subsequently associated with BrS, reflecting its genetic heterogeneity. Mutations in SCN5A are the most common genotype (>70%), but are present in only 11–28% of patients.5
The actual prevalence of BrS is unknown. It is estimated to affect around 5/10 000 individuals, but this figure may be an underestimate due to the existence of concealed electrocardiographic forms.6,7
Despite its autosomal dominant pattern, BrS is 8–10 times more prevalent in males except in children, in whom the gender distribution is similar. Clinical presentation tends to be more severe in males; structural differences in ion currents and the influence of hormonal factors have been suggested as possible explanations for differences between the sexes.8
Although it may occur at any age, BrS is typically found in young adults, with a peak of incidence between the third and fourth decade of life.2 One of the interesting aspects of the case presented is that symptoms appear to have been present since childhood. Although three of the eight patients in Brugada and Brugada's original description were children, knowledge of the condition at pediatric ages is limited1 and presentation in this age-group is uncommon. Studies in Japanese populations estimate a prevalence among children of 0.0098%, much lower than in adults (0.12%).9,10 In a study of 30 children with BrS by Probst et al., more than half were diagnosed following family screening.11 Data on BrS at pediatric ages in Portugal are scarce. Santos et al. analyzed 122 members of a Portuguese family based on an index case with a new SCN5A mutation and identified nine asymptomatic children, three of whom had diagnostic ECG, among the 40 carriers of the mutation.12
According to the report of the Heart Rhythm Society/European Heart Rhythm Association Second Consensus Conference on BrS, diagnosis requires the presence of type 1 ECG pattern, spontaneous or induced by sodium channel blockers, together with at least one of the following: documented ventricular fibrillation (VF), polymorphic ventricular tachycardia (VT), a family history of sudden cardiac death at <45 years old, coved-type ECGs in family members, inducibility of VT or VF with programmed electrical stimulation, syncope, or nocturnal agonal respiration. Brugada syndrome is definitively diagnosed when a type 1 pattern is observed in at least two right precordial leads.2 However, Richter et al. subsequently argued that revision of these criteria should be considered, after observing that individuals with a coved-type pattern in only one right precordial lead have a similar arrhythmic risk to those with diagnostic pattern in more than one.13 In the case presented, the diagnosis of BrS was based on the presence of a spontaneous type 1 pattern and a personal history of recurrent syncope.
Besides the type 1 pattern, two other ECG patterns suggestive but not diagnostic of BrS have been described: type 2 (saddleback appearance with a high takeoff ST-segment elevation of ≥2 mm, a trough displaying ≥1 mm ST elevation, and then either a positive or biphasic T wave) and type 3 (with either a saddleback or coved appearance but with an ST-segment elevation of <1 mm). A diagnosis of BrS should only be made when type 2 or 3 can be converted to type 1 pattern.2
It should be borne in mind that the ECG pattern can be dynamic, fluctuating over time between diagnostic and non-diagnostic (types 2 and 3 or normal) patterns, as seen in our patient. In a study by Veltmann et al. of 310 ECGs in 43 patients with BrS, only 35% initially presented with a type 1 pattern, and in 47% a diagnostic ECG was only documented during pharmacologic challenge. In half of the patients there were fluctuations between diagnostic and non-diagnostic ECG patterns and only one presented with a consistently diagnostic pattern.14 The results of this study highlight the importance of serial ECG recordings for correct phenotyping and risk stratification.
Various modulating factors may underlie this electrocardiographic variability over time by unmasking concealed phenotypes, and can also affect patients’ susceptibility to arrhythmic events, including a wide range of medications, alcohol or cocaine toxicity, autonomic nervous system changes, a febrile state, and potassium or calcium imbalances.15
Increasing awareness of the importance of body temperature as a modulator of arrhythmic risk in BrS has followed the publication of various isolated case reports, including in Portugal, of presentation in the context of febrile states.16–19 Barra et al. highlighted the role of fever in a case report in which after flecainide testing and programmed electrical stimulation were negative, the diagnostic phenotype was unmasked by fever.18 The molecular mechanisms behind this link are complex and poorly understood; several authors have suggested that in some SCN5A mutations elevated body temperature aggravates inactivation of sodium channels.20,21 Such modulation has been demonstrated in other conditions, including epilepsy triggered by fever.22 Amin et al. found that 18% of symptomatic events in patients with BrS occur in febrile states. They compared ECG patterns during fever and in apyrexia in 24 patients who had presented type 1 Brugada pattern during fever, which persisted in normothermia in only one.23 Patients with BrS, particularly those with a history of symptoms during febrile states, should be alerted to the need for rigorous control of body temperature during fever episodes. The effect of body temperature on the electrocardiographic and clinical phenotype is very clear in the case presented. In the absence of drugs or potassium imbalance, the type 1 pattern can be unmasked in the presence of fever but disappear completely in apyrexia, and patients may become symptomatic in febrile states, as shown by our patient's history of recurrent syncope in this case. Fever is a common trigger of arrhythmic episodes in individuals with BrS at pediatric ages, due to the frequency of infections in children; half of syncopal episodes in this age-group are estimated to occur during fever.11 In the case presented the arrhythmic event that induced the symptoms was not documented, since the patient was not under ECG monitoring during the syncopal episodes.
An ICD is currently the only proven effective treatment in BrS.2 Risk stratification for SCD is central to selection of candidates for the device in order to maximize its potential benefit. However, risk stratification is difficult due to the wide spectrum of presentation, ranging from asymptomatic individuals to those with syncope, nocturnal agonal respiration and SCD.
Aborted SCD is the first manifestation of BrS in 6–21% of patients.24–27 Probst et al., analyzing 1029 individuals with BrS in the multicenter FINGER registry, found that the cardiac event rate was very high (7.7%/year).24 The benefit of ICD implantation for secondary prevention is thus inarguable and is a class I recommendation.2
In 17–31% of BrS patients the diagnosis is made following etiologic study of syncope, which is generally secondary to self-limited episodes of polymorphic VT.24–27 Brugada et al. found that 19% of patients with a history of syncope presented SCD during follow-up.25 However, some authors report a lower incidence of arrhythmic events in this subgroup, of around 6% (1.9%/year in the FINGER registry).24–27 This difference may be due to selection bias, since the FINGER registry appears to include more severe patients. In BrS a history of syncope is a marker of arrhythmic risk, but this is also influenced by the presence of a type 1 ECG, either spontaneous or induced by sodium channel blockers. Previous syncope together with spontaneous type 1 pattern is a strong predictor of SCD and is thus an indication for an ICD (class I2 or IIa28 recommendation).2,25–27 This was the case in our patient and so he received an ICD as primary prevention. In a study of the natural history of BrS, Priori et al. found that 10% of their study population had a history of syncope and spontaneous type 1 ECG, and of these, 44% suffered SCD.26
ConclusionIn etiologic investigation of syncope or SCD in the context of a febrile state, BrS should always be considered in the differential diagnosis, and therefore an ECG is mandatory during the fever, especially after a symptomatic event. A diagnostic pattern may only be documented during the fever, a reflection of the dynamic electrocardiographic and clinical character of the condition.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martins J, Braga C, Arantes C, et al. Síncope em contexto febril – caso clínico de síndrome de Brugada. Rev Port Cardiol. 2014;33:801.e1–801.e6.