Arrhythmias during pregnancy pose a dilemma for the treating physician. Most antiarrhythmic drugs are classified as category C in the FDA labeling system during pregnancy. We describe the case of a pregnant woman who presented syncope due to drug-refractory supraventricular tachycardia who underwent catheter ablation without the use of fluoroscopy.
As arritmias na gestação geram um dilema para o médico que assiste o paciente. A maioria das drogas antiarrítmicas possuem indicação classe C durante a gestação. Aqui fazemos o relato de mulher na gestação que apresentou TSV, sincopal, refratária ao tratamento ao qual foi submetida a ablação por cateter sem o uso de fluoroscopia.
Sustained arrhythmias are relatively rare during pregnancy (2–3/1000 pregnant women investigated for palpitations); when present, supraventricular forms are the most common.1 Paroxysmal supraventricular tachycardia (SVT) presents a high rate of recurrence in pregnancy (20% in those with a history of tachyarrhythmia). In some cases, due to the physiological changes resulting from pregnancy, including increased cardiac output and reduced vascular resistance, SVT may be poorly tolerated.1
Arrhythmias during pregnancy pose a dilemma for the treating physician. Most antiarrhythmic drugs are classified as category C in the US Food and Drug Administration (FDA) labeling system during pregnancy, with the exception of sotalol (category B).2 All category C drugs cross the placental barrier and can have adverse effects on the fetus. Catheter ablation is accordingly the procedure of choice for drug-refactory, poorly tolerated SVT in pregnant women.3 However, catheters are normally visualized inside the heart by the use of ionizing radiation (X-rays), which is teratogenic and has a cumulative effect. Exposure during an electrophysiological study is approximately 25 mGy/min, resulting in a mean total exposure of 0.07 Gy.4,5 Doses higher than 0.10–0.20 Gy are associated with teratogenic effects; this figure is close to that used in one hour of fluoroscopy.5
We describe the case of a pregnant woman who presented syncope due to drug-refractory SVT who underwent catheter ablation without the use of fluoroscopy.
Case reportA 33-year-old woman, gravida 4, 26 weeks pregnant, with no known history of disease or family history of sudden death, was transferred to our department after being admitted for syncope due to paroxysmal SVT. She reported taking propanolol and sotalol prescribed during pregnancy following the first episode of SVT. Because of the severity of the clinical presentation and symptoms refractory to therapy, including class III antiarrhythmics, it was decided to treat her arrhythmia invasively. After discussing the risks and benefits with the patient, a zero-radiation approach was adopted.
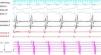
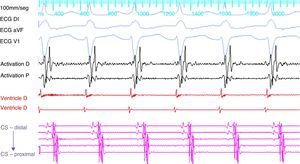
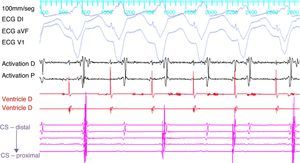
ProcedureThe patient arrived at the electrophysiology laboratory in a fasting state and was sedated with propofol and fentanyl. The baseline ECG showed sinus rhythm with normal atrioventricular (AV) conduction and no ventricular preexcitation. Fetal echocardiography was performed during the procedure for real-time monitoring of the effects of sedation and of the tachyarrhythmia on fetal dynamics. The introducer sheaths were introduced via three venous punctures and the EnSite NavX three-dimensional mapping system (St. Jude Medical, USA) was used to advance the catheters from the femoral region to the right atrium. Using only the three-dimensional mapping system, the coronary sinus and the right ventricle were catheterized and another catheter was positioned in the region of the His bundle, which was tagged with several catheter shadows to protect the AV node. Programmed atrial stimulation at a cycle length of 600 ms and extrastimuli at 340 ms reproducibly induced SVT with eccentric retrograde atrial activation that was earlier at the distal electrodes of the coronary sinus catheter (Figure 1). A ventricular extrastimulus in the refractory His bundle reproducibly terminated the SVT without atrial activity, demonstrating that it was an AV reentrant tachycardia via a left lateral accessory pathway.
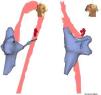
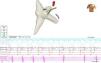
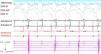
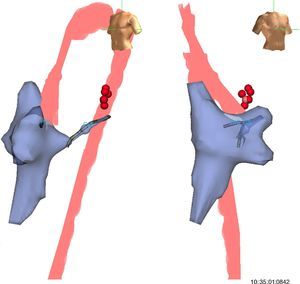
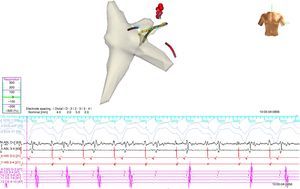
A 4-mm ablation catheter was then introduced via the femoral artery and advanced to the left ventricle under the guidance of the EnSite NavX system (Figure 2). It was not necessary to map the geometry of the left ventricle since the geometry of the coronary sinus was used to define the mitral annulus. Radiofrequency energy was applied under ventricular pacing to the region with the earliest atrial activation; after 1 s ventriculoatrial dissociation was observed, demonstrating that accessory pathway conduction had been abolished (Figures 3 and 4). Attempts to induce SVT following ablation were unsuccessful. Total procedure time was 140 min and fluoroscopy time was zero. The post-procedure echocardiogram showed no complications in either mother or fetus.
Left and right oblique views showing the geometry of the right atrium, right ventricle, coronary sinus and aorta used to position the ablation catheter. The geometry of the left ventricle was not recreated, the geometry of the coronary sinus being used to guide the catheter. The red points indicate the sites with earliest activation for radiofrequency application.
Ablation catheter positioned in the left lateral region; retrograde conduction has been terminated after radiofrequency (RF) application. The geometry illustrated is of the right atrium, right ventricle and coronary sinus. Red spheres indicate the region where RF application terminated accessory pathway conduction.
There are few cases in the literature describing ablation of tachyarrhythmias during pregnancy without the use of fluoroscopy; in most reports X-rays were used at some point in the procedure.5,6,7 However, the development of non-fluoroscopic mapping systems has opened up the possibility of pregnant patients undergoing invasive procedures without exposure to ionizing radiation. We recently published our experience with a zero radiation policy in our department8; although there were no pregnant women in the initial series, the methodology in the case presented was exactly the same.
Despite the advantages of mapping systems, they do not remove all risk of procedure-related complications. Our decision to adopt an invasive approach was prompted by the severity of the patient's clinical presentation (syncope) and her multiple emergency admissions before ablation. A recent review of management of arrhythmias in pregnancy supports our decision.9
This was the first case of SVT ablation in a pregnant woman since our department adopted a zero-radiation policy. Positioning of the catheters and three-dimensional mapping of the cardiac chambers were performed entirely under the guidance of the EnSite NavX system to ensure optimal safety of both mother and fetus.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Leiria TLL, Pires LM, Kruse ML, de Lima GG. Tratamento de taquicardia supraventricular sincopal durante a gestação sem uso de raios-X: relato de caso. Rev Port Cardiol. 2014;33:805.e1–805.e5.