An attenuated heart rate recovery (HRR) response after exercise testing is a robust predictor of mortality. Regular exercise can enhance various physiological parameters. Studies indicate that participation in a cardiac rehabilitation program can improve heart rate recovery. The aim of this study was to analyze changes in functional capacity and autonomic modulation in patients following a cardiac rehabilitation program.
MethodsBetween 2009 and 2014, 248 individuals were assessed through exercise testing, at baseline and after six months of participation in a cardiac rehabilitation program. The exercise test was performed on a treadmill using a ramp protocol. The first minute of active recovery was standardized at a speed of 1.5 mph and slope of 2.5%. The degree of parasympathetic modulation was assessed by the difference between peak exercise heart rate and heart rate at one minute of recovery. The subjects were divided into two groups according to pre-training HRR (≤12 bpm and >12 bpm).
ResultsExercise training resulted in a similar increase in metabolic equivalent values in both groups, but only the HRR ≤12 bpm group showed improvement after training (F=16.13; p<0.001), with a mean increase from 7.4±3.69 bpm to 13.0±9.74 bpm.
ConclusionsThe cardiac rehabilitation program had a positive impact in a group that had both low functional capacity and reduced parasympathetic activity, producing a favorable effect on these recognized prognostic markers.
A resposta atenuada da recuperação da frequência cardíaca após o teste de exercício é um robusto preditor de mortalidade. A prática regular de atividade física é capaz de aprimorar diversos parâmetros fisiológicos. Estudos indicam que a participação em programa de reabilitação cardíaca pode melhorar a recuperação da frequência cardíaca. Assim, o objetivo deste estudo foi avaliar as modificações na capacidade funcional e na modulação autonómica de participantes de um programa de reabilitação cardíaca.
MétodosEntre 2009-2014, 248 indivíduos foram avaliados, através do teste de exercício, antes e após seis meses de participação em programa de reabilitação cardíaca. O teste de exercício foi realizado em esteira rolante, aplicando-se o protocolo em rampa. A recuperação foi ativa e o primeiro minuto da recuperação foi padronizado, com velocidade de 1,5 mph e inclinação de 2,5%. A intensidade da modulação autonómica foi avaliada através da diferença entre a frequência cardíaca do pico do exercício e a do primeiro minuto da recuperação. Os indivíduos foram divididos em dois grupos, de acordo com a recuperação da frequência cardíaca pré-treinamento (RFC≤12 e RFC>12).
ResultadosO treinamento promoveu aumento similar na capacidade funcional de ambos os grupos. No entanto, apenas o grupo RFC≤12 mostrou acentuação na recuperação da frequência cardíaca (F=16,13; p<0,001), aumentando, em média, de 7,4±3,69 bpm para 13,0±9,74 bpm.
ConclusõesO programa de reabilitação cardíaca foi capaz de atuar favoravelmente em um grupo que possuía, simultaneamente, baixa capacidade funcional e reduzida atividade autonómica parassimpática, interferindo efetivamente nestes dois marcadores de prognóstico.
A slow decrease in heart rate (HR) after the end of incremental exercise is associated with reduced cardiac parasympathetic autonomic modulation and higher all-cause mortality.1,2
Supported by previous studies,1,2 Cole et al.3 demonstrated that an attenuated heart rate recovery (HRR) response after peak exercise is a robust predictor of mortality. A more recent study, using a reversible anticholinesterase agent during exercise, confirmed that parasympathetic activity is the main reason for variations in HR fall in the recovery period.4
Since then, several other studies have confirmed that individuals with an attenuated HRR response at one minute after exercise testing present a greater risk of death, independent of their exercise capacity, severity of coronary artery disease (CAD) and left ventricular function, even in patients with no cardiovascular symptoms.5–7 Most studies use a fall in HR of 12 bpm between peak exercise and one minute of recovery as the cut-off when assessing parasympathetic modulation, a figure endorsed by the Brazilian Society of Cardiology in its latest guidelines on exercise testing.8,9 A decrease of >12 bpm represents a normal response, while a fall of ≤12 may indicate parasympathetic dysautonomia.
Patients with cardiovascular disease undergoing a cardiac rehabilitation (CR) program, based mainly on regular exercise, show clear functional improvement with a favorable impact on prognosis.10 Previous studies have shown that the exercise component of a CR program improves HRR after peak exercise.11–16 However, Currie et al.17 found no effect on HRR after 12 weeks of high-intensity interval and moderate-intensity endurance exercise training in patients with CAD.
Against this background, the aim of the present study was to analyze the effects of a CR program on functional capacity and autonomic modulation, bearing in mind the strong prognostic impact of these variables.
MethodsThe study population consisted of all patients with stable CAD who completed at least six months of a supervised CR program at a tertiary cardiology center between May 2009 and December 2014. Data collected from medical records included baseline and post-CR exercise test results, use of negative chronotropic drugs and diagnosis of diabetes, together with demographic and anthropometric data. Patients with permanent atrial fibrillation and those with pacemakers were excluded, since these can affect HRR response.
Patients were assessed through exercise testing immediately before and after participation in the CR program. The test was performed on a treadmill using an individualized ramp protocol designed to achieve peak exercise in 10 min. There was an active recovery period of at least 5 min. The first minute of recovery was standardized at a speed of 1.5 mph and slope of 2.5%, as in Cole et al., which defined a decrease in HR of ≤12 bpm from peak exercise to one minute after the cessation of exercise as a marker of poor prognosis.3
In the present study, exercise tolerance was assessed in metabolic equivalents (METs) and HRR response in bpm in CAD patients participating in a CR program based on exercise training.
A Centurion-200 treadmill (Micromed®) linked to a computer with ERGO PC® software (Micromed) was used for the tests, with 13-lead electrocardiographic monitoring.
The supervised exercise program consisted of two or three weekly sessions lasting 60–75 min. Each session included continuous aerobic exercise lasting 30–40 min, followed by resistance training and flexibility exercises.
During the aerobic component, patients’ exercise was monitored in order to keep their HR within the target range determined by exercise testing, which defines the effective level of training and conditioning while maintaining a margin of safety. This was set at 60–80% of peak exercise HR, using the Karvonen formula. In patients with ischemia, a value of 10 bpm below the ischemic threshold was used.
In all cases, the target HR was adapted according to the modified Borg scale of perceived exertion, which enables the intensity of exercise to take account of the patient's fitness level on any given day.
The initial volume and intensity of resistance training, and their progression, were determined in accordance with international guidelines.18–20
Exercise testing and the CR program were performed in the same tertiary cardiology center.
Ethical considerationsThis research project was conducted in accordance with Brazilian National Health Council Resolution 466/12 and complementary resolutions, and with the 1988 Brazilian Code of Medical Ethics (articles 122–130). The research protocol was approved by the center's Research Ethics Committee. Since the study was based on a retrospective analysis of medical records with no intervention in the patients, who had been referred for CR by their physicians, the Ethics Committee waived the requirement for informed consent, while ensuring the anonymity of the subjects and confidentiality of their data.
Statistical analysisThe study population was divided into two groups according to baseline HRR at one minute: ≤12 bpm and >12 bpm. Analysis of variance of four factors (HRR group, time, presence of diabetes, medication) and their interactions was used to assess the effect of exercise training. Inclusion of the latter two factors was designed to control for their possible confounding effect. The analysis was applied for each of the variables under study (METs and HRR), differences being considered significant for p<0.05. The Tukey-Kramer test was used when necessary to identify differences.
ResultsThe baseline characteristics of the study population of 248 individuals are shown in Table 1, divided into two groups according to their pre-training HRR.
Baseline characteristics of the study population.
| HRR ≤12 bpm n (%) | HRR >12 bpm n (%) | Total n (%) | |
|---|---|---|---|
| Gender | |||
| Male | 48 (87.3) | 148 (76.7) | 196 (79.0) |
| Female | 7 (12.7) | 45 (23.3) | 52 (21.0) |
| Age (years) | |||
| ≤55 | 14 (25.5) | 63 (32.6) | 77 (31.0) |
| 56–65 | 19 (34.5) | 80 (41.5) | 99 (39.9) |
| >65 | 22 (40.0) | 50 (25.9) | 72 (29.0) |
| Mean (SD) | 63.2 (10.83) | 59.2 (8.65) | 60.1 (9.31) |
| BMI (kg/m2) | |||
| ≤25 | 12 (21.8) | 44 (22.8) | 56 (22.6) |
| >25 | 43 (78.2) | 149 (77.2) | 192 (77.4) |
| Mean (SD) | 27.9 (4.11) | 27.6 (3.78) | 27.7 (3.85) |
| Diabetes | |||
| No | 32 (58.2) | 137 (71.0) | 169 (68.1) |
| Yes | 23 (41.8) | 56 (29.0) | 79 (31.9) |
| Medication | |||
| None | 1 (1.8) | 13 (6.7) | 14 (5.6) |
| Beta-blocker | 42 (76.4) | 167 (86.5) | 209 (84.3) |
| CCB | 1 (1.8) | 7 (3.6) | 8 (3.2) |
| Amiodarone | 1 (1.8) | 1 (0.5) | 2 (0.8) |
| Propafenone | 1 (1.8) | 0 (0.0) | 1 (0.4) |
| Beta-blocker + digoxin | 3 (5.5) | 2 (1.0) | 5 (2.0) |
| Beta-blocker + CCB | 0 (0.0) | 1 (0.5) | 1 (0.4) |
| Beta-blocker + amiodarone | 3 (5.5) | 0 (0.0) | 3 (1.2) |
| Beta-blocker + sotalol | 2 (3.6) | 2 (1.0) | 4 (1.6) |
| Beta-blocker + ivabradine | 1 (1.8) | 0 (0.0) | 1 (0.4) |
| HR, mean (SD) | |||
| Baseline | 70.9 (13.53) | 68.4 (12.18) | 69.0 (12.52) |
| Peak | 114.9 (20.41) | 133.7 (21.03) | 129.5 (22.29) |
BMI: body mass index; CCB: calcium channel blocker; HR: heart rate; HRR: heart rate recovery; SD: standard deviation.
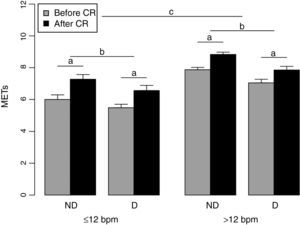
Figure 1 shows the pre- and post-training METs for the two groups. The HRR >12 bpm group had higher MET values than the HRR ≤12 bpm group, both before and after CR (F=50.02; p<0.001). However, both groups showed significant improvement in METs after CR (F=224.70; p<0.001). The absence of significant interaction between the factors HRR group and time (F=2.96; p=0.087) indicates that the two groups had similar gains in MET values following exercise training. The presence of diabetes had a significant effect on METs (F=14.59; p<0.001), with diabetic individuals presenting lower values than those without diabetes, independently of HRR and time. No other interactions were observed, nor any significant effect of medication.
Pre- and post-training metabolic equivalent values in the two groups. D: diabetic; METs: metabolic equivalents; ND: non-diabetic; CR: cardiac rehabilitation; ≤12 bpm: group with baseline heart rate recovery ≤12 bpm; >12: group with baseline heart rate recovery >12 bpm; a: significant difference before and after CR; b: significant difference between non-diabetic and diabetic individuals; c: significant difference between groups.
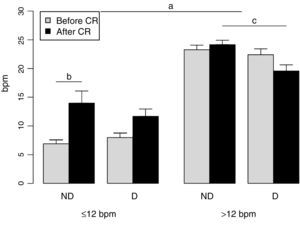
Figure 2 shows that there were significant differences between the two groups in terms of HRR (F=143.41; p<0.001). No effect of training was seen in the group with HRR >12 bpm, as shown by the significant interaction between group and time (F=16.13; p<0.001), and the presence of diabetes had only a marginal effect. Although in the HRR ≤12 bpm group only those without diabetes achieved significant improvement in HRR following CR (F=5.27; p=0.026), those with diabetes and parasympathetic dysautonomia also showed a tendency to improve; the fact that this was not significant was probably due to the small number in this group (n=23). Overall, HRR normalized in the HRR ≤12 bpm group, from 7.4±3.69 bpm to 13.0±9.74 bpm. Use of negative chronotropic agents was similar in the two groups and this variable had no influence on the effect of training on HRR (F=1.43; p=0.233).
Pre- and post-training heart rate recovery response in the two groups. D: diabetic; ND: non-diabetic; CR: cardiac rehabilitation; ≤12 bpm: group with baseline heart rate recovery ≤12 bpm; >12: group with baseline heart rate recovery >12 bpm; a: significant difference between groups; b: significant difference before and after CR; c: significant difference between non-diabetic and diabetic individuals.
There is irrefutable scientific evidence that regular exercise improves many physiological parameters, including functional capacity and peak oxygen uptake.21 The aim of the present study was to assess changes in functional capacity and HRR following an exercise-based CR program.
With regard to functional capacity, our results show that improvement was independent of initial autonomic function. Both groups improved following exercise training, with no statistical difference. It should be noted that the HRR ≤12 bpm group presented significantly worse aerobic condition on baseline assessment, reflecting greater clinical severity based on two different parameters. Following training, this group went from a condition associated with worse prognosis to one close to that considered low risk according to the literature (6.97±1.65 METs as against 7 METs in the South American Guidelines for Cardiovascular Disease Prevention and Rehabilitation22). While slightly lower than recommended, the change is significant, since every 1-MET increase in exercise capacity is associated with a 12% improvement in survival.23
Our results are in agreement with previous studies that demonstrated the positive impact of exercise training on autonomic function, as reflected in improved HRR.11,13–16 Only Currie et al.17 found no effect on HRR after 12 weeks of high-intensity interval and moderate-intensity endurance exercise training, despite improvement in physical fitness; however in their study, unlike in ours and others, the individuals assessed did not present dysautonomia prior to training.
In this respect, our results are similar to those of Streuber et al.,12 who showed that HRR improved or normalized in individuals with autonomic dysfunction at the beginning of a CR program, while those with HRR initially within the normal range remained so, with no additional gain. In our study, those who presented parasympathetic dysautonomia at initial assessment achieved a mean increase in HRR from 7.4±3.69 bpm to 13.7±9.74, thus going from a value considered pathological to one considered normal.
Despite a more than 70% improvement in HRR, 29 individuals in the HRR ≤12 bpm group remained in that range, while HRR normalized in the other 26 patients, corresponding to 47.3% of the group. This is similar to the figure reported by Jolly et al.,15 who analyzed 544 patients and found that 41% had normal HRR after rehabilitation.
Normalization of HRR is an important effect of exercise training, as various studies have shown that an attenuated HRR response following exercise testing is a predictor of mortality.3,5–7 A considerable proportion of our study population had normal HRR after CR. This is significant, since individuals with autonomic dysfunction before training who achieve normal HRR after training appear to have similar survival rates to those with normal HRR at baseline and after cardiac rehabilitation.15
The present study has certain limitations. It was retrospective and as such has the limitations inherent to this type of study. However, our sample was representative of real-world patients since the only exclusion criteria were conditions that would make it impossible to accurately assess HR, and thus no patients were excluded based on disease severity or the presence of comorbidities.
Assessment of functional capacity was limited to conventional exercise testing, and thus peak oxygen uptake was only estimated, and temporary technical problems prevented analysis of expired gases and oxygen uptake. However, this did not affect the main finding of our study since HR behavior during the test would have been the same, irrespective of whether oxygen uptake was measured directly or estimated.
Finally, we did not use HR variability to assess autonomic modulation, but rather a parameter that has been used in various studies for this purpose – HRR response at one minute of recovery.1–3
ConclusionsOur results show that cardiac rehabilitation has an important impact on prognosis. The exercise-based CR program played a positive role in a high-risk group that had both low functional capacity and reduced parasympathetic activity, producing a markedly favorable effect on these recognized prognostic markers.
Ethical responsibilitiesProtection of people and animalsThe authors state that the procedures followed were in accordance with regulations established by the heads of the Clinical and Research Ethics Commission and according to the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Funding sourcesThe present study received no external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
SS receives a postdoctoral grant from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Please cite this article as: Nascimento PMC, Vieira MC, Sperandei S, Serra SM. Atividade física supervisionada melhora a modulação autonómica de participantes de reabilitação cardíaca. Rev Port Cardiol. 2016;35:19–24.