Dilated cardiomyopathy is the most common form of cardiomyopathy and the main cause of cardiac transplantation in children and in adults. Infants and children have a wider spectrum of etiologies, hampering their identification. The most frequent initial manifestation of dilated cardiomyopathy is symptomatic heart failure during exercise or at rest (although many patients are asymptomatic). Some causes are potentially reversible, therefore the investigation should be carefully planned and immediately performed after diagnosis. In most children no cause is identified, which limits the targeted therapeutic approach and therefore the effectiveness of the treatment.
The authors present a case of dilated cardiomyopathy secondary to renovascular hypertension diagnosed in an infant with 3.5 month-old, highlighting the etiological investigation, treatment and evolution.
The authors present this case emphasising the fact that the arterial hypertension diagnose in infants is not always easy, questioning the current recommendations relating to an initial evaluation on blood pressure. We postulate that the assessment of blood pressure in newborns can detect early renovascular hypertension (and even other cardiovascular diseases) and help prevent the development of deleterious effects, including fatal episodes.
A miocardiopatia dilatada é a forma mais comum de miocardiopatia e a principal causa de transplante cardíaco em idade pediátrica e em adultos. Os lactentes e as crianças apresentam etiologias de espectro mais alargado, embora a sua identificação seja mais difícil. A manifestação inicial mais frequente da miocardiopatia dilatada é a insuficiência cardíaca sintomática, em esforço ou repouso (embora muitos pacientes sejam assintomáticos). Como algumas das causas da miocardiopatia dilatada são potencialmente reversíveis, a investigação deve ser cuidadosamente planeada e feita imediatamente após o diagnóstico. Na maioria das crianças não é identificada uma causa, o que limita a abordagem terapêutica dirigida e, portanto, a eficácia do tratamento implantado.
Os autores apresentam um caso de miocardiopatia dilatada secundária a hipertensão arterial renovascular, diagnosticada numa lactente de 3,5 meses, e destacam a investigação etiológica, o tratamento instituído e a sua evolução.
Os autores salientam que o diagnóstico de hipertensão arterial no lactente nem sempre é fácil e questionam as atuais recomendações para início da medição da pressão arterial. Postulamos que a avaliação da pressão arterial em recém-nascidos pode detetar hipertensão arterial renovascular precoce (e mesmo outras doenças cardiovasculares) e ajudar a prevenir o desenvolvimento de efeitos deletérios, inclusive episódios fatais.
Dilated cardiomyopathy (DCM) is the most common form of cardiomyopathy and the main cause of cardiac transplantation in children and adults.1 DCM is a heterogeneous group of disorders with dilatation of the heart chambers and myocardial dysfunction as common phenotypes. Although these phenotypes occur in both adults and children, the underlying etiologies and prognosis differ depending on the age group.2 The most common etiology in adults is coronary artery disease, although cases associated with inflammatory conditions, toxins (medication, alcohol and illegal drugs) and genetic defects have also been described. There is a broader spectrum of etiologies in infants and children, although these causes are more difficult to identify.3 It is idiopathic in 66% of cases, and myocarditis (46%) and neuromuscular disease (26%) are among the most common known causes.3 Inborn errors of metabolism and malformative syndromes are also causes in infants under 1 year of age.4
One North American study3 reported that the annual incidence of DCM in children was 0.57 cases per 100000, and that it was more common in males (0.66 vs. 0.47/100000) and in infants than in older children (4.40 vs. 0.34/100000). Previously, a Finnish study found a DCM incidence in children of 0.4 cases per 100000 and a prevalence of 2.6 cases per 100000 in Finland.5 In the United Kingdom, the incidence rate is 0.87/100000 in individuals >16 years of age.6
The most common initial manifestation in children with DCM is symptomatic heart failure, with effort or at rest (although many patients are asymptomatic).4,7 Ventricular arrhythmias, atrioventricular block, syncope and sudden death may also occur.4
Because some of causes of DCM are potentially reversible, an extensive panel of investigations should be planned and performed immediately after diagnosis.1 Nonetheless, most children do not have a known cause, which limits disease-specific therapies and, therefore, the efficacy of the treatment implemented.3
Description of the case reportFemale infant with no significant personal history except for a drop in weight-for-age percentile from one month of age (50th percentile to 15th percentile). At 3 months of age, the parents reported onset of sweating and pale lips during feeding, which gradually worsened.
At age 3.5 months, she was brought to the emergency department due to an episode of hypotonia and moaning, interpreted as a brief resolved unexplained event. Fever and recent infection were denied and she had no family history of heart disease or sudden death. Physical examination found the patient to be in good general condition, with blood pressure (BP) at 112/81 mmHg (P>99), peripheral oxygen saturation at 99%, grade II/VI systolic murmur, and no organomegaly or peripheral edema. High BP was subsequently confirmed in all four limbs, with no significant difference among values. Complete blood count was normal and a blood chemistry panel showed normal transaminases, renal function and thyroid function. Brain natriuretic peptide was found to be high (2686.7 pg/ml).
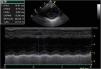
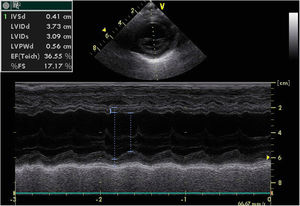
A chest X-ray revealed cardiomegaly with no pulmonary congestion. An electrocardiogram showed sinus rhythm, normal QRS axis, no pathological Q waves and inverted T wave in LI, LII and LIII. An echocardiography found dilatation of the left chambers with global left ventricular dysfunction, left ventricular ejection fraction (LVEF) 36%, left ventricular systolic function (LVSF) 17% and moderate mitral insufficiency, with coronary arteries of apparently normal origin (Figure 1).
Thus, the infant was diagnosed with DCM with heart failure and hypertension (HT). She was then hospitalized for an etiological study, and treatment with captopril and furosemide was initiated.
During the hospital stay, she underwent extensive laboratory and imaging tests to rule out metabolic, infectious and immunological pathologies. They found a slight increase in the right lobe of the liver with no hepatic cytolysis and non-specific acylcarnitine deficiency; serologies were negative for cytomegalovirus, herpes 1 and 2 viruses, Epstein-Barr virus, parvovirus B19 and toxoplasmosis; polymerase chain reaction was negative for enterovirus and adenovirus; the immunoglobulin, complement C1q, C3c and C4 fractions and haptoglobin assays were normal; and assay was negative for antinuclear antibodies, antineutrophil cytoplasmic antibodies, anticardiolipin antibodies, anti-extractable nuclear antigen antibodies and anti-double stranded DNA antibodies. A fundoscopy found no abnormalities.
Her renal function was normal, with elevated aldosterone (>150 ng/dl). Renin assay was not possible due to theoretical reasons. A renal ultrasound showed renal asymmetry with a larger left kidney and parenchymal hyperechogenicity.
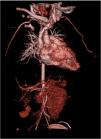
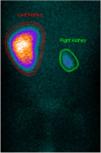
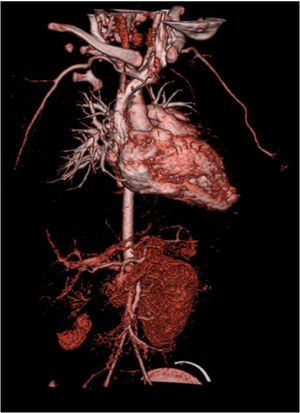
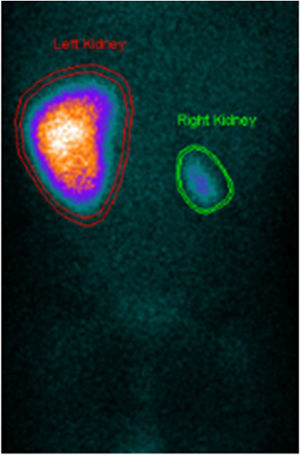
A computed tomography angiography was performed to better visualize vasculature, which revealed “diffuse stenosis of the right renal artery in its initial portion, with reduced size at its distal end. Right kidney smaller than the left, associated with reduced parenchymal thickness and marked delay in contrast uptake. Reduced caliber of inferior abdominal aorta (3.5 mm), as well as of the common iliac arteries (2 mm)” (Figure 2). The patient subsequently underwent renal scintigraphy with dimercaptosuccinic acid, which confirmed the right kidney was small and had almost complete functional exclusion (4.5%) (Figure 3).
The case was discussed then with the Vascular Surgery and Interventional Radiology departments. Given the impossibility of endovascular or surgical correction of the renal artery stenosis, nephrectomy was considered, ideally after stabilizing cardiac function.
After a 19-day hospital stay, the patient was discharged asymptomatic and with controlled BP, medicated with furosemide (1 mg/kg/day), spironolactone (2 mg/kg/day), nifedipine (1.9 mg/kg/day) and carvedilol (0.08 mg/kg/day).
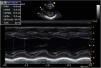
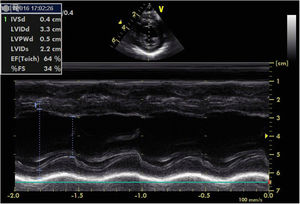
For the next six months, BP remained stable with no need to initiate new drugs, and echocardiographic progress was favorable, with gradual improvement of left ventricular function. At 10 months of age, the patient underwent right nephrectomy without complications. Since then, gradual weaning from the drugs was possible. At 11 months of age, recovery of ventricular function was confirmed (LVEF 64%, LVSF 34%); the patient continued to have a very slightly dilated left ventricle (Figure 4).
At 13 months of age, she underwent abdominal magnetic resonance angiography, which found that “infrarenal abdominal aorta and common iliac arteries are still somewhat small, but have no filling defects”. Currently, at 20 months of age, she remains asymptomatic with normal BP. She is medicated with low-dose carvedilol in monotherapy (being weaned off by 0.016 mg/kg/day). Clinical monitoring of this patient continues at the Pediatric Cardiology and Nephrology offices.
Discussion and conclusionThe authors presented this case, emphasizing that HT is not always easy to diagnose in an infant. This is because BP is difficult to measure in very young subjects and results are hard to interpret, with high values often misinterpreted as agitation. HT in infants usually appears with non-specific symptoms, such as apnea, tachypnea, prostration, irritability, feeding problems, and cardiovascular or neurological symptoms.8
From a cardiovascular perspective, in DCM, newborns and infants most often develop congestive heart failure, unlike older children and adults who develop left ventricular hypertrophy secondary to HT. As happened with our patient, improvement or resolution of DCM associated with normalizing BP has also been reported.9
Thus, diagnosis of HT and DCM in an infant should lead to a careful etiological investigation, since some causes may be treatable and can significantly improve prognosis.
Renovascular HT causes 5-10% of HT cases in children. At the time of diagnosis, patients often have evidence of target-organ damage.10 Treatment is both medical (antihypertensive agents) and surgical (endovascular treatment, surgical bypass grafting or nephrectomy).11,12 In the case described here, the patient's age, functional exclusion of the kidney and renal parenchymal atrophy meant that nephrectomy was the only possible intervention.
Finally, this case led the authors to question the current recommendations to start taking BP measurements in healthy children at 2 years of age. The authors believe that taking BP measurements in newborns could enable early detection of HT, and possibly other cardiovascular diseases, and prevent the clinical deterioration in these cases that are often associated with serious or fatal events.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Alves I, Martins T, Neves AL, Rodrigues E, Teixeira A, Afonso C, et al. Deveremos avaliar a pressão arterial no recém-nascido? Rev Port Cardiol. 2018;37:625.