A permanent pacemaker is frequently needed after transcatheter aortic valve implantation, but the available data are mainly on the CoreValve system.
ObjectiveTo evaluate the need for new permanent pacemaker after implantation of the Edwards Sapien device, as well as related factors.
MethodsWe included the first 100 patients treated with the Edwards Sapien device at our institution. Of these, 12 had a permanent pacemaker before the procedure, and thus our study population was the remaining 88 patients.
ResultsA permanent pacemaker was indicated in eight patients (9.1%) during hospitalization or at 30 days. After discharge, another four patients needed a pacemaker (at 42 days and three, 18, and 30 months). Two variables were associated with the need for pacemaker during hospitalization: previous dialysis (13% vs. 1%, p=0.042) and complete right bundle branch block before the procedure (25% vs. 5%, p=0.032). More than one month after the procedure, the characteristics associated with the need for pacemaker were plasma creatinine level (2.5±1.7 vs. 1.3±0.6 mg/dl, p=0.001) and previous myocardial infarction (50% vs. 10%, p=0.013).
ConclusionThe rate of pacemaker implantation with the Edwards Sapien device was 9.1%. Right bundle branch block and dialysis were associated with this complication.
A necessidade de um pacemaker permanente após implantação percutânea da válvula aórtica é frequente, embora os dados disponíveis estejam principalmente associados ao sistema CoreValve.
ObjetivosO objetivo foi avaliar o índice do novo pacemaker permanente, bem como todos os fatores relacionados, após a implantação do dispositivo Edwards-Sapiens.
MétodosIncluímos os primeiros 100 doentes tratados com o dispositivo Edwards-Sapiens no nosso hospital. Destes, 12 já tinham pacemaker permanente antes do procedimento, pelo que a população do estudo corresponde aos restantes 88 doentes.
ResultadosO pacemaker permanente foi indicado em oito doentes (9,1%) durante o internamento ou a 30 dias. Após a alta hospitalar, outros quatro doentes necessitaram de colocar o pacemaker (aos 42 dias e aos três, 18 e 30 meses). Duas variáveis foram relacionadas com a necessidade de colocação de pacemaker durante o internamento: diálise prévia (13 versus 1%, p=0,042) e bloqueio completo do ramo direito antes do procedimento (25 versus 5%, p=0,032). Mais do que um mês após o procedimento, as características, que foram relacionadas com a necessidade de colocação de pacemaker, foram os níveis da creatinina plasmática (2,5±1,7 versus 1,3±0,6 mg/dl, p=0,001) e enfarte do miocárdio prévio (50 versus 10%, p=0,013).
ConclusãoA necessidade de colocação de pacemaker após a implantação do dispositivo de Edwards-Sapiens foi de 9,1%. O bloqueio completo do ramo direito e a diálise foram associados a esta complicação.
The need for pacemaker (PM) secondary to severe atrioventricular (AV) conduction abnormalities is a relatively common complication after transcatheter aortic valve implantation (TAVI).1–12 This is apparently due to mechanical compression of the conduction system by the device, as the His bundle and left branch are anatomically very close to the aortic annulus and aortic valve.13,14
The need for PM implantation after TAVI is especially frequent with the self-expanding CoreValve (CV) prosthesis (Medtronic Inc., Minneapolis, MN), and therefore information about this complication is mainly available on patients treated with this device.1,2,5–11 By contrast, there are fewer data on the need for PM with the balloon-expandable Edwards Sapien (ES) valve (Edwards Lifesciences Inc., Irvine, CA).12,15
Furthermore, data on the need for PM implantation after TAVI are mainly related to the periprocedural period, whereas there is less information on longer follow-up. This is important, because patients referred for TAVI are frequently of advanced age, and may require PM implantation unrelated to TAVI.
The objective of this study was to evaluate the need for PM implantation, as well as related factors, both short- and long-term, after ES device implantation. For this purpose, we performed a long-term follow-up of the first 100 patients treated with the ES at our institution.
MethodsStudy populationThe first 100 patients treated with the ES at our institution were included in the study. In all cases, the indication was established by the heart team, with the participation of clinical cardiologists, interventional cardiologists and cardiac surgeons. Briefly, patients had symptomatic severe aortic stenosis (valve area <1 cm2) with high surgical risk and an estimated survival >1 year. Initially, a EuroSCORE >20% was required, but subsequently patients with EuroSCORE <20% and with other situations (e.g., patent left internal mammary artery grafts and porcelain aorta were accepted).
Of the 100 patients, 78 underwent TAVI by transfemoral access and 22 by transapical access. The transfemoral approach was the first choice, but transapical TAVI was performed when the iliac anatomy did not allow a safe procedure by a transfemoral approach.
TechniqueIn all cases, the procedure was performed under three-dimensional transesophageal monitoring and general anesthesia.
A femoral vein was punctured to advance a temporary pacemaker into the right ventricle, and a femoral artery was used to advance a pigtail catheter into the ascending aorta for angiographic monitoring of the procedure. After the aortic valve was crossed with the guidewire, balloon valvuloplasty under rapid pacing was performed in most cases. The prosthetic valve was also implanted under rapid pacing (180–220 bpm).
The temporary pacemaker was withdrawn 24 hours after the procedure in the absence of conduction abnormalities.
Statistical analysisThe statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± standard deviation, and compared with the Student's t test. Discrete variables were expressed as percentages (proportions), and compared with the chi-square test (with Fisher's correction when necessary). Associations were considered statistically significant with p<0.05. The incidence of PM implantation was estimated with Kaplan-Meier survival analysis.
ResultsRate of pacemaker implantationA new permanent PM was indicated before hospital discharge in eight patients. The PM was implanted between 48 and 72 hours in six cases, and in 3–5 days in two. However, 12 out of the 100 patients had a permanent PM before TAVI. Thus, the rate of new PM implantation was 9.1% (eight of 88 patients without previous PM). The indication for PM was atrial fibrillation with heart rate <50 bpm in four patients, sinus rhythm with complete AV block in three, and sinus rhythm with high-degree AV block in one.
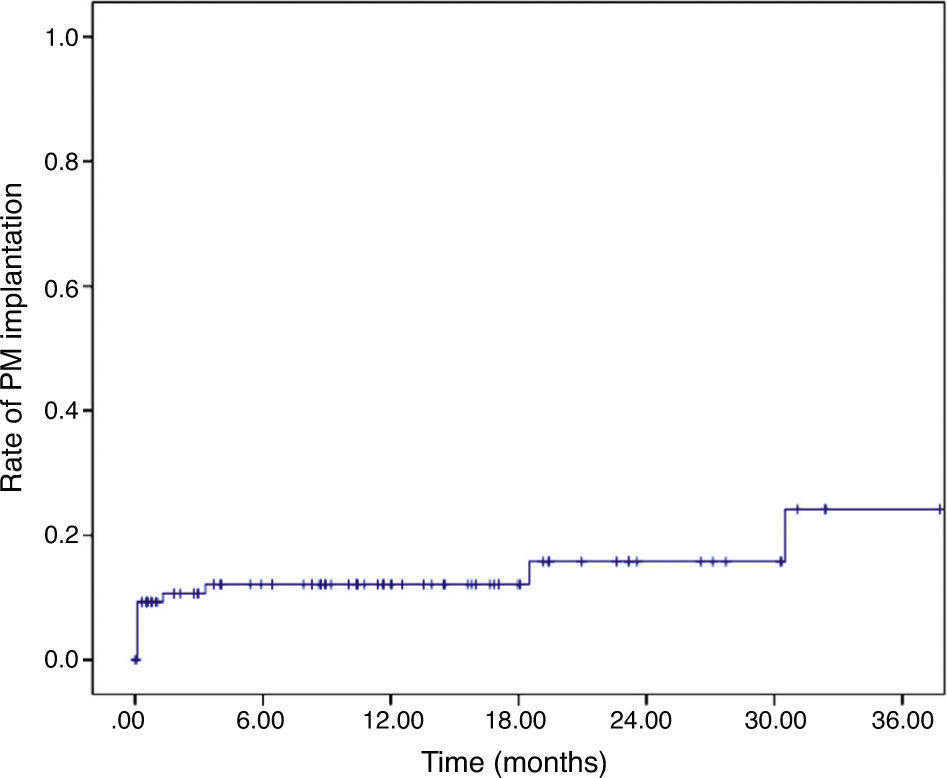
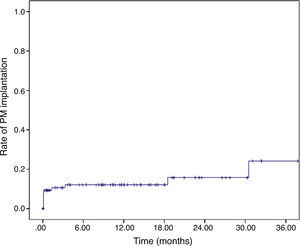
After discharge, another four patients required a permanent PM, at 42 days, three months, 18 months, and 30 months. The cumulative rate of PM implantation was 9.1±0.3%, 12.1±3.6%, 12.1±3.6%, 15.8±5.0% and 24.2±9.2% at 30 days, six months, one year, two years and three years, respectively (Figure 1).
Characteristics associated with the need for pacemaker implantationImportant variables such as age, aortic annulus diameter, prosthetic valve diameter, syncope previous to the procedure, and left ventricular ejection fraction were not associated with the need for a new PM during the first 30 days or during follow-up (Tables 1–3). Interestingly, some parameters of renal function were associated with the need for PM implantation in both the short and long term. The presence of right bundle branch block was associated with the need for PM during the first month after TAVI.
Association between baseline clinical and echocardiographic characteristics and the need for permanent pacemaker implantation during hospitalization or in the first month after TAVI.
| PM | No PM | p | |
|---|---|---|---|
| Age (years) | 82±4 | 82±7 | 0.880 |
| Female gender (%) | 63 | 58 | 0.785/1.000 |
| Weight (kg) | 74±14 | 70±13 | 0.369 |
| Height (cm) | 158±8 | 159±9 | 0.778 |
| Body mass index (kg/m2) | 30±5 | 28±6 | 284 |
| Hypertension (%) | 100 | 79 | 0.147/0.345 |
| Hypercholesterolemia (%) | 50 | 51 | 0.946/1.000 |
| Diabetes (%) | 25 | 39 | 0.444/0.705 |
| Previous infarction (%) | 0 | 13 | 0.288/0.589 |
| Previous ischemic heart disease (%) | 38 | 46 | 0.636/0.723 |
| Previous PCI (%) | 38 | 34 | 0.831/1.000 |
| Previous CABG (%) | 50 | 34 | 0.944/0.341 |
| Previous stroke (%) | 13 | 20 | 0.608/1.000 |
| Previous chronic lung disease (%) | 0 | 23 | 0.133/0.199 |
| Creatinine clearance (ml/min) | 37±22 | 48±25 | 0.327 |
| Baseline plasma creatinine (mg/dl) | 1.7±0.9 | 1.3±0.7 | 0.97 |
| Previous hemodialysis (%) | 13 | 1 | 0.042/0.175 |
| Previous bundle branch block (%) | 38 | 16 | 0.137/0.155 |
| Previous RBBB (%) | 25 | 5 | 0.032/0.091 |
| Previous LBBB (%) | 13 | 11 | 0.915/1.000 |
| Previous malignancy (%) | 0 | 4 | 0.577/1.000 |
| Previous atrial fibrillation (%) | 50 | 43 | 0.683/0.722 |
| Logistic EuroSCORE (%) | 19±9 | 16±9 | 0.331 |
| Previous syncope (%) | 13 | 16 | 0.782/1.000 |
| Transapical approach (%) | 38 | 23 | 0.366/0.398 |
| Valve prosthesis diameter (mm) | 24.9±2.2 | 24.3±1.7 | 0.367 |
| Aortic annulus diameter (mm) | 21.6±2.5 | 20.9±2.1 | 0.416 |
| Aortic valve area (cm2) | 0.66±0.20 | 0.68±0.21 | 0.789 |
| Peak transvalvular gradient (mmHg) | 77±13 | 75±23 | 0.856 |
| Mean transvalvular gradient (mmHg) | 41±9 | 46±16 | 0.316 |
| LVEF (%) | 53±16 | 57±11 | 0.358 |
CABG: coronary artery bypass grafting; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; PM: pacemaker; RBBB: right bundle branch block.
In the comparison of proportions, p values for both chi-square test and Fisher's correction are provided.
Association between baseline clinical and echocardiographic characteristics and the need for late (>1 month) permanent pacemaker implantation.
| PM | No PM | p | |
|---|---|---|---|
| Age (years) | 80±9 | 82±7 | 0.692 |
| Female gender (%) | 25 | 60 | 0.172/0.305 |
| Weight (kg) | 71±11 | 70±13 | 0.889 |
| Height (cm) | 159±13 | 159±9 | 0.900 |
| Body mass index (kg/m2) | 28±2 | 28±6 | 0.992 |
| Hypertension (%) | 50 | 82 | 0.112/0.167 |
| Hypercholesterolemia (%) | 75 | 50 | 0.328/0.617 |
| Diabetes (%) | 25 | 38 | 0.597/1.000 |
| Previous infarction (%) | 50 | 10 | 0.013/0.062 |
| Previous ischemic heart disease (%) | 75 | 44 | 0.224/0.326 |
| Previous PCI (%) | 50 | 33 | 0.492/0.603 |
| Previous CABG (%) | 75 | 35 | 0.100/0.135 |
| Previous stroke (%) | 0 | 20 | 0.317/1.000 |
| Previous chronic lung disease (%) | 25 | 20 | 0.818/1.000 |
| Creatinine clearance (ml/min) | 32±9 | 47±25 | 0.285 |
| Baseline plasma creatinine (mg/dl) | 2.5±1.7 | 1.3±0.6 | 0.001 |
| Previous hemodialysis (%) | 25 | 1 | 0.002/0.089 |
| Previous bundle branch block (%) | 25 | 18 | 0.717/0.559 |
| Previous RBBB (%) | 0 | 7 | 0.580/1.000 |
| Previous LBBB (%) | 25 | 11 | 0.379/0.388 |
| Previous malignancy (%) | 25 | 2 | 0.015/0.132 |
| Previous atrial fibrillation (%) | 25 | 44 | 0.452/0.631 |
| Logistic EuroSCORE (%) | 15±7 | 17±9 | 0.777 |
| Previous syncope (%) | 25 | 15 | 0.611/0.507 |
| Transapical approach (%) | 25 | 24 | 0.978/1.000 |
| Valve prosthesis diameter (mm) | 25.0±1.7 | 24.3±1.7 | 0.508 |
| Aortic annulus diameter (mm) | 21.6±1.8 | 21.0±2.1 | 0.622 |
| Aortic valve area (cm2) | 0.76±0.09 | 0.67±0.21 | 0.424 |
| Peak transvalvular gradient (mmHg) | 81±23 | 75±22 | 0.585 |
| Mean transvalvular gradient (mmHg) | 45±15 | 44±15 | 0.929 |
| LVEF (%) | 56±3 | 57±12 | 0.901 |
CABG: coronary artery bypass grafting; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; PM: pacemaker; RBBB: right bundle branch block.
In the comparison of proportions, p values for both chi-square test and Fisher's correction are provided.
Association between baseline clinical and echocardiographic characteristics and the need for early or late permanent pacemaker implantation.
| PM | No PM | p | |
|---|---|---|---|
| Age (years) | 81±6 | 82±7 | 0.909 |
| Female gender (%) | 50 | 59 | 0.548/0.549 |
| Weight (kg) | 73±13 | 69±13 | 0.391 |
| Height (cm) | 158±9 | 159±9 | 0.860 |
| Body mass index (kg/m2) | 29±5 | 28±6 | 0.349 |
| Hypertension (%) | 83 | 80 | 0.802/1.000 |
| Hypercholesterolemia (%) | 58 | 50 | 0.591/0.758 |
| Diabetes (%) | 25 | 40 | 0.336/0.523 |
| Previous infarction (%) | 17 | 11 | 0.533/0.621 |
| Previous ischemic heart disease (%) | 50 | 45 | 0.734/0.764 |
| Previous PCI (%) | 42 | 33 | 0.551/0.534 |
| Previous CABG (%) | 50 | 34 | 0.291/0.341 |
| Previous stroke (%) | 8 | 21 | 0.300/0.448 |
| Previous chronic lung disease (%) | 8 | 22 | 0.263/0.446 |
| Creatinine clearance (ml/min) | 37±19 | 48±25 | 0.148 |
| Baseline plasma creatinine (mg/dl) | 2.0±1.2 | 1.2±0.6 | <0.001 |
| Previous hemodialysis (%) | 17 | 0 | <0.001/0.017 |
| Previous bundle branch block (%) | 34 | 16 | 0.143/0.219 |
| Previous RBBB (%) | 17 | 5 | 0.145/0.188 |
| Previous LBBB (%) | 17 | 11 | 0.533/0.621 |
| Previous malignancy (%) | 8 | 3 | 0.312/0.359 |
| Previous atrial fibrillation (%) | 42 | 43 | 0.909/1.000 |
| Logistic EuroSCORE (%) | 18±8 | 16±9 | 0.491 |
| Previous syncope (%) | 17 | 16 | 0.938/1.000 |
| Transapical approach (%) | 33 | 22 | 0.438/0.476 |
| Valve prosthesis diameter (mm) | 24.9±2.0 | 24.3±1.7 | 0.249 |
| Aortic annulus diameter (mm) | 22±2 | 21±2 | 0.326 |
| Aortic valve area (cm2) | 0.70±0.17 | 0.67±0.21 | 0.754 |
| Peak transvalvular gradient (mmHg) | 78±16 | 75±23 | 0.628 |
| Mean transvalvular gradient (mmHg) | 42±11 | 45±16 | 0.610 |
| LVEF (%) | 54±13 | 57±11 | 0.398 |
CABG: coronary artery bypass grafting; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; PM: pacemaker; RBBB: right bundle branch block.
In the comparison of proportions, p values for both chi-square test and Fisher's correction are provided.
Underlined value refers to branch block either RBBB or LBBB.
Previous dialysis and right bundle branch block at baseline showed a statistically significant association with need for PM using the chi-square test (Table 1). However, after performing Fisher's correction, only right bundle branch block showed a tendency for a greater need for PM.
The presence of right bundle branch block at baseline was not significantly related to the need for PM more than one month after TAVI (Table 2). Nevertheless, creatinine plasma level before the procedure was associated with the need for new PM beyond the first month: 2.5±1.7 mg/dl and 1.3±0.6 mg/dl in patients with and without need for PM, respectively (p=0.001). On the other hand, a history of previous myocardial infarction was statistically associated with the need for late PM.
Considering both the first month after TAVI and long-term follow-up, there were only two characteristics associated with the need for a new PM: plasma creatinine at baseline and dialysis previous to TAVI (Table 3).
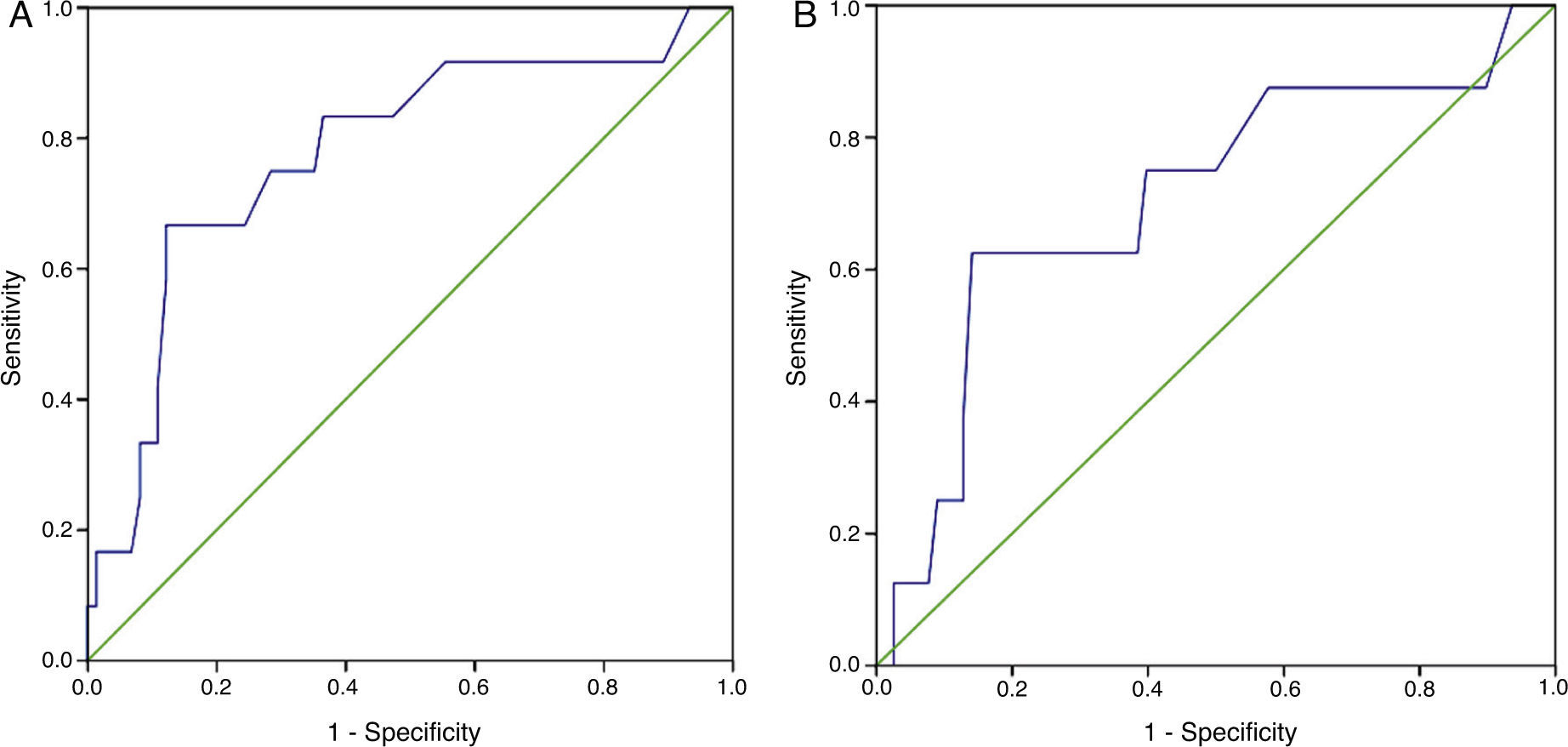
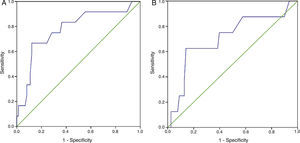
Receiver operating characteristic (ROC) curves for plasma creatinine showed an area under the curve (AUC) for need for PM during hospitalization of 0.706 (95% confidence interval [CI]: 0.500–0.912) (Figure 2A). The ROC curve showed an AUC for need for PM throughout follow-up of 0.777 (95% CI: 0.624–0.930) (Figure 2B).
DiscussionNeed for pacemaker after transcatheter aortic valve implantationSome patients undergoing TAVI need a permanent PM because of severe AV conduction disturbances,1–12,15 which appear to be related to device-induced trauma of the interventricular septum (IVS).14 During and after TAVI, new left bundle branch block is relatively frequent, secondary to conduction system damage during the procedure.16 As a result, patients with conduction abnormalities before the procedure are at higher risk of needing a permanent PM after TAVI.
Although the rate is 2.5–5 times lower with the ES than with the CV,2,5,9 4–11% of patients treated with the ES need a permanent PM.1–12 With both systems, this complication is more common than with surgical aortic valve replacement.17 In our series, 9.1% of patients treated with the ES needed a permanent PM during initial hospitalization, which is similar to the 8.8% incidence in the PARTNER trial and other studies.1–3,12,15
Beyond the first month after TAVI, some patients also needed PM implantation, but probably in these cases it was not directly due to the valve procedure. The need for PM beyond the first month after TAVI is probably related to the type of patients that are candidates for TAVI (advanced age, and high prevalence of renal failure), irrespective of the valve intervention.
The need for PM after TAVI is not associated with higher mortality after the procedure,3,9 but this complication prolongs hospital stay and can lead to complications such as pneumothorax, bleeding and infections. Moreover, PM rhythm may be associated with impaired ventricular function18 and a higher incidence of cardiac failure and cerebrovascular events during follow-up.19,20
Most of the conduction abnormalities associated with TAVI develop during the procedure (sometimes even before valve implantation)21 or soon after, but may also occur beyond the first 48 hours.22 These conduction disturbances are usually persistent, but some studies have shown that a significant proportion of patients undergoing permanent PM implantation after TAVI were not PM-dependent months after the procedure.8,11,7 This could justify more restrictive indications for permanent PM after TAVI, precluding unnecessary PM implantation by delaying the decision in some patients.23 However, our opinion is rather not to delay the indication for PM, because in most cases conduction abnormalities after TAVI are irreversible, and prolonged temporary transvenous pacing may also be associated with complications.24 Moreover, in some studies of patients treated with TAVI, those discharged with a permanent PM had a lower risk of sudden death after discharge.3
Characteristics associated with the need for permanent pacemakerAs the risk of permanent PM after TAVI is higher with the CV system,2,5,9 a number of characteristics have been identified associating this complication with the use of this device1,2,4,6,9,15,25,26: depth of implantation, use of first-generation devices, IVS thickness, left ventricular end-diastolic diameter, conduction abnormalities before the procedure, valve oversizing, age over 75, heart rate below 65 bpm before the procedure, atrial fibrillation, porcelain aorta, balloon pre-dilatation, and non-coronary cusp thickness.
In our series of patients treated with the ES, we found that both right bundle branch block and terminal chronic renal failure (on dialysis) were associated with the need for early permanent PM (during hospitalization or during the first month). Other variables, such as access route (transfemoral or transapical), age, valve diameter, aortic annulus diameter, or body mass index, were not associated with this complication.
Right bundle branch block has been associated with higher frequency of PM implantation in several series of TAVI with the CV.4,10,27,28 The AV node and left bundle branch are located near the aortic root and native aortic valve,13 while the non-coronary cusp is adjacent to the membranous portion of the IVS, which extends into a triangle bounded by the non-coronary and the right coronary cusps, where the His bundle is located. The left branch emerges in the membranous septum, crosses the fibrous trigone and passes along the left surface of the muscular septum. This is why previous right bundle branch block increases the risk of complete AV block if there is damage to the left ventricular outflow tract during TAVI.
The association between renal function and the need for PM after TAVI is less well understood. Renal failure is associated with electrolyte abnormalities that can induce rhythm and conduction disturbances. Most importantly, chronic renal failure is associated with deposition of extracellular matrix and collagen and development of fibrosis in the myocardium that may result in conduction abnormalities, arrhythmias and systolic and diastolic dysfunction.29 More severe renal dysfunction is associated with a higher prevalence of left bundle branch block and advanced AV block.30 Renal failure is also a predictor of progression to complete AV block in patients with bifascicular block,31 which is consistent with the greater need for permanent PM in longer-term follow-up that we observed late after TAVI.
Finally, in our study previous infarction was associated with the need for permanent PM more than one month after TAVI. Myocardial dysfunction occurring late after myocardial infarction may induce conduction abnormalities. Previous myocardial infarction was not, however, associated with the need for PM during the first month after TAVI.
ConclusionsMost data on the need for PM implantation after TAVI have been obtained with the self-expanding CV device. In our study, performed with the balloon-expandable ES prosthetic valve, 9.1% of patients needed a permanent PM during initial hospitalization or the first month after the procedure. Right bundle branch block and renal function were associated with the need for PM after TAVI. On the other hand, patients treated with TAVI are at high risk of needing PM in the long term, probably not directly due to TAVI, but probably due to the characteristics of this patient population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.