The adipocytokines visfatin and omentin have a direct effect on inflammation and endothelial injury. The expression of visfatin is closely associated with the expression of proinflammatory cytokines. Omentin has an anti-inflammatory effect and is inversely associated with coronary artery disease (CAD). The slow coronary flow phenomenon is an angiographic finding characterized by delayed distal vessel opacification in the absence of significant epicardial coronary disease. The pathophysiology of SCF has not been clearly identified, although multiple abnormalities including endothelial dysfunction, atherothrombosis and inflammation have been reported. However, the relationship between visfatin, omentin and SCF is still unknown. In this study, we aimed to investigate the relationship of these adipocytokines with SCF.
MethodsThe study included slow coronary flow (n=45) and normal coronary flow (n=55) subjects, according to the corrected TIMI frame count, who underwent angiography in the catheterization laboratory of Duzce University. Statistical analyses were performed with SPSS version 12.
ResultsVisfatin levels were significantly higher in patients with SCF than in controls (p<0.001). Plasma omentin levels were lower in the SCF group than in controls, although without statistical significance. Visfatin, gender and platelet count were significant predictors of SCF in multivariate logistic regression analysis (OR 0.748, 95% CI 0.632–0.886, p=0.01; OR 30.016, 95% CI 4.355–206.8, p=0.01; OR1.028, 95% CI 1.006–1.050, p=0.011, respectively).
ConclusionAdipocytokines such as visfatin and omentin may play a role in the pathogenesis of coronary slow flow.
A visfatina e o omentin são adipocitocinas e têm um efeito direto sobre a inflamação e a lesão endotelial. A expressão da visfatina está intimamente associada com a expressão de citoquinas pró-inflamatórias. O omentin tem efeito anti-inflamatório e está inversamente associado com a doença coronária (DC). O fenómeno do fluxo coronário lento (FCL) é um achado angiográfico caracterizada por atraso de opacificação distal na ausência de doença coronária epicárdica significativa. A fisiopatologia do FCL não está claramente identificada, apesar de terem sido relatadas várias alterações, incluindo disfunção endotelial, aterotrombose e inflamação. No entanto, a relação entre visfatina, o omentin e a FCL ainda é desconhecida. Neste estudo, procurou-se investigar essas relações das adipocitocinas com o FCL.
MétodosNo estudo foram incluídos indivíduos com fluxo coronário lento (n = 45) e fluxo coronário normal (n = 55) de acordo com a contagem corrigida de quadros TIMI, que necessitavam de angiografia no laboratório de cateterismo da Universidade de Duzce. As análises estatísticas foram realizadas com o programa estatístico SPSS 12.
ResultadosOs níveis de visfatina foram significativamente mais altos em pacientes com FCL do que nos do grupo controle (p < 0,001). Os níveis plasmáticos de omentin foram menores no grupo FCL do que nos controles, embora sem significado estatístico. Numa análise de regressão logística multivariada a visfatina, o género e a contagem de plaquetas foram definidos como preditores significativos da FCL (respetivamente, OR 0,748, IC 95% 0,632-0,886, p = 0,01; OR 30,016, 95% CI 4,355-206,8, p = 0,01 e OR 1.028, IC 95% 1,006-1,050, p = 0,011).
ConclusãoAs adipocitocinas, por exemplo a visfatina e o omentin, podem desempenhar um papel na patogénese do fluxo coronário lento.
The slow coronary flow (SCF) phenomenon was identified as a discrete clinical entity in 19721 in which distal opacification of the coronary artery is delayed on angiography in the absence of significant coronary artery disease. Another feature of SCF is its frequent occurrence in association with more widespread vascular abnormalities. SCF is a frequent finding in patients presenting with acute coronary syndrome, usually unstable angina.
Although some underlying etiologies such as abnormally high microvascular resistance and widespread atherosclerosis of the coronary arteries have been proposed, the exact pathophysiological mechanism of this phenomenon remains unclear. In some patients, diffuse atherosclerosis or diffuse calcifications have been identified on intravascular ultrasound.2–4 Other factors that may cause SCF are abnormalities in the coronary microcirculation, microvascular endothelial dysfunction and inflammation.5
Inflammation is controlled by various hormones and cytokines. Adipose tissue secretes a variety of adipocytokines, including leptin, adiponectin, visfatin, TNF-α, IL-6 and omentin, and it is considered an endocrine organ due to its effects on metabolism in several organs and systems.6
The inflammatory cytokine visfatin plays a key role in delayed neutrophil apoptosis in sepsis. It is highly enriched in the visceral fat of both humans and mice, and its plasma levels increase during the development of obesity,7 as well as being elevated in patients with type 2 diabetes, suggesting that measurement of plasma visfatin can be a useful tool for understanding metabolic diseases.8,9
Omentin is a recently identified adipokine that is selectively expressed in visceral adipose tissue.10,11 Recent studies have shown that omentin levels are negatively correlated with acute coronary syndrome and stable angina pectoris.12 Plasma omentin levels correlate negatively with systolic blood pressure, hemoglobin A1C, body mass index (BMI) and total cholesterol levels, and positively with HDL cholesterol.13
The relationship between plasma levels of these adipocytokines and SCF has not been investigated. We aimed to investigate the relations of visfatin and omentin plasma levels with SCF.
MethodsForty-five consecutive patients who had undergone coronary angiography in Duzce University School of Medicine Cardiology Clinic between June 2012 and January 2013 and were found to have slow coronary flow were included in the study. Fifty-five consecutive patients with completely normal coronary arteries were recruited as the control group. The indications for coronary angiography were stable angina and positive treadmill test. Patients with a history of congestive heart failure, CAD including spasm, plaque, or ectasia, valvular heart disease, hyperthyroidism, chronic obstructive pulmonary disease and patients with acute coronary syndrome were excluded from the study. Those with a BMI of 30 or higher were considered obese. Coronary angiography was performed using the standard Judkins technique and the results were analyzed by at least two cardiologists. Slow coronary flow was defined as a corrected TIMI frame count >27 with a correction factor of 1.7 for the left anterior descending coronary artery (LAD) based upon images acquired at 30 frames/s. The study protocol was approved by the Ethics Committee of Duzce University and all subjects provided written consent.
Calculation of TIMI frame countWe used the TIMI frame count method developed by Gibson et al. for objective evaluation of coronary blood flow.14 Briefly, the frame count was calculated as the number of angiographic frames elapsed until the contrast material arrived at the pre-specified distal marker of the individual coronary artery. Because of its greater length, the corrected TIMI frame count for the LAD was calculated by dividing the counted value by 1.7. The first frame was considered to be that at which >70% lumen opacification with antegrade filling was noted. The frame in which dye opacification reached a certain distal landmark in each vessel was accepted as the final frame. Normal TIMI frame count values are 36.2±2.6, 22.2±4.1, 20.4±3 for LAD, left circumflex and right coronary artery, respectively.14
Serum visfatin and omentin measurementsPlasma visfatin concentrations were measured in duplicate with a human visfatin (COOH-terminal) enzyme immunometric assay (Phoenix Pharmaceuticals, Belmont, CA) performed on an Alisei Quality System (SEAC Radim Group, Pomezia, Italy). Assay sensitivity was 2 ng/ml, and interassay and intra-assay coefficients of variation were <10% and <5%, respectively. Plasma omentin-1 levels were determined using an enzyme-linked immunosorbent assay (ELISA) (USCN Life Science Inc., Cologne, Germany). Intra- and interassay coefficients of variation for the omentin ELISA were <6.7% and <9.1%, respectively.
Statistical analysisStatistical Package for the Social Sciences software (SPSS 12, Chicago, IL, USA) was used for the analysis. Descriptive parameters were expressed as mean±standard deviation or as percentages. The Kolmogorov-Smirnov test was used to test for normality of distribution. Abnormally distributed variables were compared using the Mann-Whitney U test. Independent sample t tests and Pearson's chi-square tests were used to analyze the differences in means and proportions between groups. Comparisons between groups were tested with ANOVA. Logistic regression analysis was used to assess predictors of slow coronary flow. Variables with p<0.1 by univariate analysis were included in the multivariate logistic regression analysis model and respective odds ratios (OR) and 95% confidence intervals (CI) were calculated. Continuous variables were expressed as means±standard deviation. A p value <0.05 was considered significant.
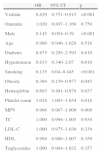
ResultsForty-five patients (33 men and 12 women, mean age 59.4±11.5 years) were diagnosed as having SCF. The control group was made up of 55 individuals (30 men and 25 women, mean age 57.8±9.9 years) with normal coronary vessels. The demographic and clinical characteristics of the study groups are shown in Table 1.
Demographic and clinical characteristics of the study groups.
| Controls (n=55) | SCF group (n=45) | p | |
| Male, n (%) | 30 (54) | 33 (70) | <0.001 |
| Age (mean±SD) | 57.8±9.3 | 59.4±9.2 | 0.82 |
| Diabetes, n (%) | 12 (21) | 10 (22) | 0.832 |
| Hypertension, n (%) | 22 (40) | 20 (44) | 0.814 |
| Smoking, n (%) | 11 (20) | 22 (48) | <0.001 |
| Obesity, n (%) | 17 (30) | 27 (60) | 0.007 |
| Hyperlipidemia, n | 6 | 5 | 0.88 |
| Family history of CHD, n | 9 | 7 | 0.17 |
| Statin use, n | 5 | 5 | 0.756 |
| BMI (kg/m2) (mean±SD) | 27.5±1.8 | 28.6±1.8 | 0.010 |
BMI: body mass index; CHD: coronary heart disease; SCF: slow coronary flow.
Rates of obesity and smoking and BMI values were significantly higher in the SCF group than in the control group (p<0.05) (Table 1). As shown in Table 2, there were no differences in laboratory variables, including white blood cell count, lipid parameters, and creatinine, between the two groups. However, hemoglobin, platelet count and mean platelet volume were higher in SCF patients than in controls (p<0.05).
Comparison of laboratory findings in the study groups.
| Controls (n=55) | SCF group (n=45) | p | |
| WBC count, 103/μl | 5.8±1.9 | 6.2±1.2 | 0.51 |
| Hemoglobin, g/dl | 11.4±1.5 | 12.1±1.2 | 0.04 |
| Hematocrit, % | 34.9±4.5 | 36.3±4.5 | 0.11 |
| Platelet count, 103/μl | 238.4±33.6 | 266.2±54.2 | 0.01 |
| MPV, fl | 7.14±1.03 | 7.75±0.95 | 0.009 |
| ALT, U/ml | 18.5±7.48 | 18.7±4.8 | 0.909 |
| AST, U/ml | 17.5±6.2 | 18.9±3.3 | 0.497 |
| TC, mg/dl | 174.6±39.7 | 177.6±42.6 | 0.743 |
| LDL-C, mg/dl | 88.4±32.8 | 95.4±34.2 | 0.361 |
| HDL-C, mg/dl | 41.5±25.2 | 38.7±12.9 | 0.562 |
| Triglycerides, mg/dl | 157.7±61 | 159.2±93.3 | 0.931 |
| BUN, mgr/dl | 18.6±11.1 | 15.91±4.4 | 0.18 |
| Creatinine, mg/dl | 0.96±0.19 | 0.99±0.4 | 0.71 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; MPV: mean platelet volume; SCF: slow coronary flow; TC: total cholesterol; WBC: white blood cell.
Visfatin and omentin levels are summarized in Table 3. Visfatin levels were significantly higher in the SCF group (p<0.001), while omentin levels were similar between the two groups (p=0.75).
In univariate analysis, visfatin, gender, smoking, obesity, hemoglobin, platelet count and mean platelet volume were significantly correlated with SCF (Table 4). Among these, visfatin, gender and platelet counts were found to be significant predictors of SCF in multivariate logistic regression analysis (Table 5).
Predictors of slow coronary flow in univariate analysis.
| OR | 95% CI | p | |
| Visfatin | 0.829 | 0.751–0.915 | <0.001 |
| Omentin | 1.020 | 0.897–1.160 | 0.759 |
| Male | 0.145 | 0.054–0.39 | <0.001 |
| Age | 0.986 | 0.946–1.028 | 0.518 |
| Diabetes | 0.875 | 0.295–2.593 | 0.810 |
| Hypertension | 0.833 | 0.340–2.07 | 0.610 |
| Smoking | 0.135 | 0.04–0.445 | <0.001 |
| Obesity | 0.394 | 0.159–0.977 | 0.045 |
| Hemoglobin | 0.693 | 0.491–0.978 | 0.037 |
| Platelet count | 1.018 | 1.003–1.034 | 0.018 |
| MPV | 0.998 | 0.987–1.009 | 0.009 |
| TC | 1.000 | 0.994–1.005 | 0.934 |
| LDL-C | 1.000 | 0.975–1.030 | 0.339 |
| HDL | 0.994 | 0.980–1.007 | 0.359 |
| Triglycerides | 1.000 | 0.984–1.032 | 0.357 |
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; MPV: mean platelet volume; TC: total cholesterol.
Adipocytokines such as leptin, resistin, visfatin and omentin are currently being investigated as potential drug targets in type 2 diabetes, lipid metabolism disorders, endothelial dysfunction and inflammatory diseases in general.
SCF is considered a variant of CAD, but its exact cause has not been fully elucidated. Several mechanisms have been proposed, including microvascular vasomotor dysfunction, diffuse atherosclerosis, small-vessel inflammation, and endothelial dysfunction.15 In this study, we aimed to investigate the relationship between coronary slow flow and the adipocytokines visfatin and omentin.
Visfatin is the most recently discovered of the family of adipocytokines and has been described as an inflammatory cytokine. Increased expression of visfatin mRNA has been observed in inflammatory conditions including atherosclerosis and inflammatory bowel disease.16 Visfatin is regarded as an inflammatory mediator, localized to foam cell macrophages within unstable atherosclerotic lesions, that potentially plays a role in plaque destabilization.17 Plasma visfatin concentrations increase in patients with overweight/obesity, type 2 diabetes, metabolic syndrome and cardiovascular disease, and circulating visfatin level is positively associated with insulin resistance.7,9 However, the relationship between SCF and serum visfatin levels has not been investigated. Our findings show that visfatin levels is significantly higher in patients with SCF (p<0.001).
Omentin is a novel fat depot-specific adipokine that was identified in 2003 from a visceral omental adipose tissue cDNA library and has anti-inflammatory effects.18 The omentin gene is located in the 1q22-q23 chromosomal region, which has been linked to type 2 diabetes in several populations, suggesting that it may be a candidate gene for type 2 diabetes susceptibility in humans.19,20
Several clinical studies have shown that serum omentin is significantly decreased in obese patients and patients with polycystic ovary syndrome or diabetes.21,22 In addition, circulating omentin levels are negatively correlated with metabolic risk factors, including BMI, waist circumference, and insulin resistance.21,22 A negative correlation of omentin has also been shown with increased carotid intima-media thickness in patients with metabolic syndrome.23 De Souza Batista et al. showed that omentin gene expression was decreased with obesity and decreased omentin levels were associated with increasing obesity and insulin resistance.24 However, no previous studies have clarified the relationship of serum omentin with SCF.
In our study the mean plasma level of omentin was lower in the SCF group than in controls, although the difference was not statistically significant (p=0.754).
Study limitationsA possible limitation of the study was the small study population. The odds ratio of male gender was very large, possibly due to the small sample size. Further studies with larger study populations are required.
ConclusionAccording to our results, the adipocytokines visfatin and omentin may play a role in the pathogenesis of slow coronary flow. In order to clarify their relationship with SCF and to investigate their potential as drug targets in endothelial dysfunction and inflammatory diseases, these molecules should be studied in larger groups.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.