Balloon pulmonary angioplasty (BPA) is an alternative therapy in patients with inoperable chronic thromboembolic pulmonary hypertension (CTEPH) or residual/recurrent pulmonary hypertension (PH) after surgery. The aim of this study was to assess the short-term efficacy and safety of a BPA program.
MethodsThis prospective single-center study included all BPA sessions performed in CTEPH patients between 2017 and 2019. Clinical assessment including WHO functional class, plasma biomarkers, 6-min walk test (6MWT) and right heart catheterization was performed at baseline and six months after the last BPA session.
ResultsA total of 57 BPA sessions were performed in 11 CTEPH patients (64% with inoperable disease, 82% under pulmonar vasodilator therapy). Nine patients completed both the BPA program and a minimum six-month follow-up period. There were significant improvements in WHO functional class (p=0.004) and 6MWT (mean increase of 42 m; p=0.050) and a trend for significant hemodynamic improvement: 25% decrease in mean pulmonary artery pressure (mPAP) (p=0.082) and 42% decrease in pulmonary vascular resistance (PVR) (p=0.056). In the group of patients with severely impaired hemodynamics (three patients with mPAP >40 mmHg), the reduction was significant: 51% in mPAP (p=0.013) and 67% in PVR (p=0.050). Prostacyclin analogs and long-term oxygen therapy were withdrawn in all patients. Minor complications were recorded in 25% of patients. There were no major complications or deaths.
ConclusionsA BPA strategy on top of pulmonary vasodilator therapy further improves symptoms, exercise capacity and hemodynamics with an acceptable risk-benefit ratio in patients with inoperable CTEPH or residual/recurrent PH after surgery.
A angioplastia pulmonar por balão (BPA) constitui uma alternativa terapêutica na hipertensão pulmonar tromboembólica crónica (HPTEC) inoperável ou hipertensão pulmonar (HP) residual/recorrente após cirurgia. O objetivo deste estudo foi avaliar a eficácia e segurança a curto prazo do programa de BPA.
MétodosEstudo prospetivo em centro único, que incluiu todas as sessões de BPA em doentes com HPTEC entre 2017 a 2019. Realizou-se avaliação da classe funcional da OMS, biomarcadores plasmáticos, teste de marcha de 6 minutos (TM6M) e cateterismo cardíaco direito no início e seis meses após a última sessão.
ResultadosForam realizadas 57 sessões de BPA em 11 doentes com HPTEC (64% doença inoperável, 82% sob terapêutica vasodilatadora pulmonar). O programa de BPA foi concluído com um seguimento mínimo de seis meses em nove doentes. Houve uma melhoria significativa na classe funcional OMS (p=0.004), no TM6M (aumento médio de 42 metros; p=0.050) e uma tendência para melhoria hemodinâmica significativa: queda de 25% na pressão da artéria pulmonar média (PAPm; p=0.082) e 42% na resistência vascular pulmonar (RVP; p=0,056). No subgrupo de doentes que apresentavam critérios hemodinâmicos de gravidade (três doentes com PAPm>40 mmHg), a redução foi significativa: 51% na PAPm (p=0,013) e 67% na RVP (p=0,050). Foram suspensos os análogos da prostaciclina e a oxigenoterapia de longa duração em todos os doentes. Foram registadas complicações minor em 25%. Não foram registadas complicações major ou mortes.
ConclusõesUma estratégia de BPA em doentes sob terapêutica vasodilatadora pulmonar melhora os sintomas, a capacidade de exercício e a hemodinâmica com uma relação risco-benefício aceitável em doentes com HPTEC inoperável ou HP residual/recorrente após cirurgia.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a progressive chronic disease that is characterized by obstruction of the pulmonary arterial vasculature by organized thrombotic material, leading to fibrosis, thickening of the intima, vascular remodeling and plexiform lesions that cause increased resistance and pressure in the pulmonary circulation, severe pulmonary hypertension (PH), right heart overload, right ventricular failure and death.1–6 Prognosis is poor if only treated medically, with one-, three- and five-year survival of 90.2%, 78.4% and 64.5%, respectively.7 Surgical pulmonary endarterectomy (PEA) is the first-line treatment for patients with CTEPH and, when performed in experienced centers, is associated with in-hospital mortality of <5% and leads to hemodynamic and functional improvement, with good long-term survival.8,9 However, fewer than 60% of patients are considered eligible for surgery.10–12 Reasons for patients to be rejected for surgery include more distal location of the disease (technically inoperable) and patient-related factors (technically operable but with a poor risk/benefit ratio for surgery due to comorbidities).10 Furthermore, up to 50% of operated patients suffer residual or recurrent PH, worsening morbidity and mortality.12–14 Medical treatment with riociguat, a soluble guanylate cyclase stimulator, improves hemodynamics and exercise capacity in these patients but is not curative.15–17 In this context, balloon pulmonary angioplasty (BPA), an endovascular procedure for dilating stenotic or obstructed pulmonary arteries, has emerged as an alternative therapeutic option for CTEPH patients who are unsuitable for surgery or who have recurrent or persistent PH after PEA.12
After the first case report, published in 1988,18 the first case series on BPA as a treatment for CTEPH, by Feinstein et al., was published in 2001.19 The technique proved to be effective, lowering mean pulmonary artery pressure (mPAP) and improving functional class, but had a high rate of complications, particularly fatal reperfusion pulmonary injury and need for mechanical ventilation,19 and was abandoned for a decade.
The technique re-emerged in 2012 when centers in Japan20–22 published three series reporting greater safety as well as clinical and pulmonary hemodynamic improvements. These favorable results were confirmed in a multicenter Japanese registry23 that included 308 patients with CTEPH treated by BPA in a total of 1408 sessions in seven centers. Significant hemodynamic improvements were seen, with lower pulmonary vascular resistance (PVR) in over 50% of patients, and overall survival at 1–3 years comparable to surgery (96.8% and 94.5%, respectively).
In Europe, the number of centers starting BPA programs has grown considerably in recent years.24–28 The largest case series in a single French center was recently published, including 184 patients participating in a total of 1006 BPA sessions; it confirmed significant short-term improvements in symptoms, exercise capacity and hemodynamics, as well as the safety of the procedure and its long-term efficacy, with three-year survival of 95.1%.29
No published data are currently available on the Portuguese experience with BPA. The present study aims to present the results of the initial experience in our center and to assess the short-term symptomatic, functional and hemodynamic benefits, as well as the safety, of BPA in the treatment of CTEPH patients.
MethodsPopulation selectionAll patients with CTEPH who were ineligible or contraindicated for EAP were included after being assessed for operability by a multidisciplinary team from a surgical center experienced in EAP, as recommended by the current guidelines.12 Patients with persistent or recurrent PH following EAP were also included.
Participants underwent a complete diagnostic study including clinical history and assessment of comorbidities, pulmonary ventilation-perfusion scan, computed tomography angiography of the thorax with biplane reconstructions, digital subtraction pulmonary angiography, and right catheterization. All patients gave their written informed consent to the study, which was approved by the ethics committee of Hospital Garcia de Orta and conformed to the Declaration of Helsinki.
Assessment before and after balloon pulmonary angioplastyAll patients underwent clinical assessment at the time of diagnosis of CTEPH (for patients treated by PEA, this assessment was performed after surgery), before the first BPA session (baseline) and six months after the last session (follow-up). Clinical assessment included World Health Organization (WHO) functional class, 6-min walk test (6MWT), serum N-terminal pro-brain natriuretic peptide (NT-proBNP), arterial blood gas analysis and complete right heart catheterization, the latter to measure mPAP, PVR and cardiac output by the thermodilution method.
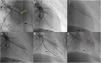
Balloon pulmonary angioplasty techniqueBPA was performed in accordance with the previously published methodology used in our center30 (Figure 1).
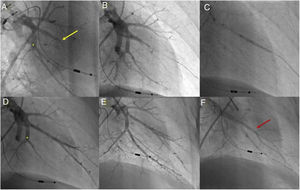
Balloon pulmonary angioplasty of segment A7 + 8 of the left lower lobe. (A) Selective pulmonary angiography demonstrating total occlusion of the A8 segment (yellow arrow) and a web at the bifurcation of segment 7 (*); (B) passage of a Whisper MS guidewire (Abbot Vascular, Santa Clara, CA, USA), through the occlusion in segment A8; (C) vessel dilation with a semicompliant 4.0/20 Pantera Pro balloon (Biotronik SE & Co KG, Berlin, Germany); (D) selective pulmonary angiography showing a good final result in segment A8 and segment A7 not yet treated (*); (E) following dilation of segment A7 at the level of the web (*), a good final angiographic result is achieved, with increased arterial flow; (F) venous return observed (red arrow) on final selective angiography of segment A7 + 8 documenting pulmonary flow grade 3.
During the program, outpatient therapy was continued, including anticoagulation and specific pulmonary vasodilators. Multiple BPA sessions were required to fully treat every patient. In the first sessions, priority was given to treating less complex lesions such as stenoses, webs and subocclusive lesions. Total occlusions and lesions in tortuous vessels were treated less frequently. In patients with more severely impaired hemodynamics (mPAP >40 mmHg and/or PVR>7 Wood units [WU]), an undersized balloon was used in the vessel, particularly in the initial sessions, and functional assessment techniques such as pressure-wire-guided BPA were used.31 The number of segments treated in each session varied according to the patient’s hemodynamic severity, procedure time (<60 min fluoroscopy), and the quantity of iodinated contrast used (≤300 ml, depending on baseline renal function).
Whenever possible, each patient underwent two BPA sessions in one hospitalization. Subsequent sessions were scheduled at intervals of 3–4 weeks until mPAP and/or PVR fell below 25 mmHg and 4 Woods unit (WU), respectively, under pulmonary vasodilator therapy and/or until all accessible lesions had been treated.
Safety analysisBPA-related complications were defined as (1) angiographic vascular lesions, including vessel dissection and perforation); (2) hemoptysis; (3) pulmonary injury, divided into five grades using a previously published classification system32; (4) vascular access site complications including hematoma and seroma; and (5) contrast nephropathy, defined as a blood creatinine increase of 25% or of 0.5 mg/dl over the value before the procedure.33 To facilitate early detection of potential complications, all patients underwent laboratory testing and chest X-ray 24 and 48 h post-procedure.
Major procedure-related complications were defined as pulmonary injury of grade 3 or higher or other complications requiring intervention, need for non-invasive positive airway pressure ventilation, mechanical ventilation or extracorporeal membrane oxygenation (ECMO), or complications resulting in the patient’s death.
Complications were adjudicated by an interventional cardiologist and two experts in PH.
Efficacy analysisThe success of the BPA program was evaluated at six months by invasive assessment of pulmonary vascular hemodynamics (mPAP and PVR) and of clinical parameters indicating exercise tolerance (WHO functional class and 6MWT).
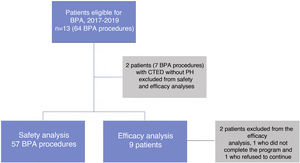
Complications (safety data) were assessed in all BPA procedures in patients with CTEPH. Efficacy data were assessed in all CTEPH patients who completed all scheduled BPA sessions and who underwent follow-up catheterization six months after the last BPA session (Figure 2).
Statistical analysisStatistical analyses were performed using IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA). Categorical variables were presented as number and percentage, and continuous variables as mean ± standard deviation for normal distributions or median and interquartile range for non-normal distributions. The chi-square test, Fisher’s exact test and the McNemar test were used to compare categorical variables, and the paired Student’s t test or the Wilcoxon non-parametric test was used to compare paired samples of continuous variables. The analysis assumed a significance level of 5%.
ResultsPopulation characteristicsBetween December 2017 and November 2019 (23 months), a total of 64 BPA sessions were carried out in our center in 13 patients, 57 of which were in patients diagnosed with CTEPH. The other seven sessions were for patients with chronic thromboembolic disease, without PH, and were therefore excluded from the analysis.
The 57 sessions analyzed were carried out in 11 patients diagnosed with CTEPH, whose mean age was 66±12 years, and 73% of whom were female. A history of acute venous thromboembolism was reported in eight patients (73%). At the time of CTEPH diagnosis, all patients had limitation of physical activity (WHO functional class ≥II in 100% and class IV in two). Mean NT-proBNP was 538 (192–1255) pg/ml and mean 6MWT distance was 326±138 m. All patients were under anticoagulant therapy, 82% were taking pulmonary vasodilators (including two patients taking prostacyclin analogs), and two patients (18%) were under long-term oxygen therapy.
On the hemodynamic assessment before the first BPA session, mPAP was 33.5±11.8 mmHg and PVR was 5.0±3.2 WU. Three patients (27%) presented severely impaired hemodynamics (mPAP >40 mmHg), with mPAP 50.3±0.6 mmHg and PVR 8.5±3.6 WU.
The main indication for inclusion in the BPA program was non-eligibility for PEA surgery (seven patients, 65%); four of these patients had mainly distal lesions, two had an unfavorable risk/benefit ratio for PEA, and one refused surgery. Among the inoperable patients, median time between diagnosis of CTEPH and the first BPA session was 10 (3–58) months. Four patients (36%) were included due to residual or recurrent PH following surgery; median time between PEA and the first BPA session was 58 (19–78) months.
The baseline clinical and hemodynamic characteristics (assessed before the first BPA session) of the study population are summarized in Table 1.
Baseline clinical, hemodynamic and procedural characteristics (11 patients, 57 procedures).
| Clinical characteristics | |
| Age, years | 66±12 |
| Female, n (%) | 8 (73%) |
| History of acute VTE, n (%) | 8 (73%) |
| WHO functional class I/II/III/IV, n (%) | 0/9 (82%)/2 (18%)/0 |
| 6MWT, m | 407±62 |
| NT-proBNP, pg/ml | 204 (118-602) |
| PaO2, mmHg | 67±21 |
| Oxygen therapy, n (%) | 2 (18%) |
| Hemodynamic characteristicsa | |
| Systolic PAP, mmHg | 54.1±20.0 |
| Diastolic PAP, mmHg | 21.6±5.8 |
| mPAP, mmHg | 33.5±11.8 |
| Mean PAD, mmHg | 5.4±2.9 |
| PCWP, mmHg | 9.5±2.2 |
| PVR, WU | 5.0±3.2 |
| Cardiac output, l/min | 5.0±1.1 |
| Cardiac index, l/min/m2 | 2.8±0.6 |
| SvO2, % | 66.0±8.6 |
| Pulmonary vasodilator therapy | 9 (82%) |
| Soluble guanylate cyclase stimulators, n (%) | 7 (64%) |
| Endothelin receptor antagonists, n (%) | 4 (36%) |
| Phosphodiesterase type 5 inhibitor, n (%) | 2 (18%) |
| Prostacycline analogs, n (%) | 2 (18%) |
| Medication (none/monotherapy/dual/triple), n (%) | 2 (18%)/5 (46%)/3 (27%)/1 (9%) |
| Indications for BPA | |
| Ineligible for PEA, n (%) | 7 (64%) |
| Residual PH after surgery, n (%) | 4 (36%) |
| Procedural characteristics | |
| BPA sessions, n | 57 |
| Sessions per patient, n | 5.4±1.9 |
| Segments treated per session | 2.4±1.0 |
| Vessels treated per session, n | 3.9±1.7 |
| Balloons per session, n | 3.8±1.1 |
| Lung treated (right/left/both), n | 35/16/6 |
| Type of lesion per session, n | |
| Web | 2.7±1.7 |
| Stenosis | 0.3±0.6 |
| Subtotal occlusion | 0.5±0.7 |
| Total occlusion | 0.3±0.7 |
| Contrast per session, ml | 267±78 |
| Mean fluoroscopy time, min | 61±15 |
6MWT: 6-min walk test; BPA: pulmonary balloon angioplasty; mPAP: mean pulmonary artery pressure; PaO2: partial pressure of oxygen; PCWP: pulmonary capillary wedge pressure; PEA: pulmonary endarterectomy; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation; VTE: venous thromboembolism; WHO: World Heath Organization.
Of the total of 57 sessions, each patient underwent a mean of 5.4±1.9 sessions. The number of vessels treated was 220 (mean 3.9±1.7 per session) and the number of segments treated was 135 (mean 2.4±1.0 per session). A mean of 21.1±8.8 vessels and 10.0±2.4 segments were treated per patient. Webs, subtotal occlusions, total occlusions and stenoses were treated in 153 (69.5%), 31 (14.1%), 19 (8.6%) and 17 (7.7%), respectively, of the vessels treated. The right lung (n=35; 61.4%) and particularly the A10 segment of the lower right lobe (n=20, 35.1%) were most frequently addressed. Intravascular imaging was used in five sessions (8.8%), while functional assessment using a pressure wire was used to guide 10 procedures (17.5%), mostly in cases of severely impaired hemodynamics (mPAP and PVR in these procedures were 41.7±9.0 mmHg and 6.5±2.6 WU, respectively). Mean procedure time was 124±30 min, mean fluoroscopy time (including right heart catheterization) was 61±15 min, and mean contrast volume used was 267±78 ml per session.
Safety analysis (procedural complications)The rate of procedure-related complications was 24.6% (Table 2). Vascular lesions were observed in six sessions (10.5%): three distal perforations by the guidewire accompanied by mild hemoptysis, and three dissections. Two of the three perforations required prolonged balloon inflation, which resulted in complete resolution of the lesion. The remaining perforation and the dissections resolved without need for percutaneous or surgical intervention. Hemoptysis was recorded in a total of six sessions (10.5%) and pulmonary injury, classified as mild (grade 2) and not requiring invasive mechanical ventilation or ECMO, occurred in three sessions (5.3%). Vascular access site complications and contrast nephropathy occurred in only one session each (1.8%). No patients presented acute radiation-induced dermatitis. No major procedure-related complications were noted and there were no periprocedural deaths.
Complications (57 sessions).
| Event | Total, n (% sessions) |
|---|---|
| Vascular lesion | 6 (10.5) |
| Perforation | 3 (5.3) |
| Dissection | 3 (5.3) |
| Hemoptysis | 6 (10.5) |
| Pulmonary injury | 3 (5.3) |
| Grade ≥3 | 0 |
| Mechanical ventilation | 0 |
| ECMO | 0 |
| Vascular access site complication | 1 (1.8) |
| Contrast nephropathy | 1 (1.8) |
| Other | 2 (3.5) |
| In-hospital mortality | 0 |
| Overall complicationsa | 14 (24.6) |
ECMO: extracorporeal membrane oxygenation.
Of the nine patients who completed the BPA program and follow-up, 89% were under pulmonary vasodilator therapy. The median time between diagnosis of CTEPH and the first BPA session was 55 (8–69) months.
Pulmonary vasodilator therapy enabled a reduction in the number of patients in advanced functional classes, with two patients in WHO functional class IV improving to class III and two in class III improving to class II. Thus, before the beginning of the BPA program, there were no patients in class IV (Table 3). There were also improvements in 6MWT distance (mean increase of 60 m; p=0.208), although without statistical significance; two patients in advanced functional classes were unable to perform this test at the beginning of the program. Median NT-proBNP levels fell from 538 to 204 pg/ml (p=0.156), although again without statistical significance. Regarding hemodynamics, there were significant reductions of about 20% in mPAP (43.4±11.3 vs. 34.8±12.5 mmHg, p=0.018) and of 48% in PVR (10.2±4.5 vs. 5.3±3.4 WU, p=0.001). Pulmonary vasodilator therapy also significantly increased cardiac index (2.2±0.6 vs. 2.7±0.6 l/min/m2, p=0.041).
Clinical and hemodynamic characteristics (9 patients).
| Variable | Initial (time of diagnosis) | Baseline (beginning of BPA program) | 6-month follow-up | Initial vs. baseline p | Baseline vs. 6-month follow-up p |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| WHO functional class I/II/III/IV | 0/5/2/2 | 0/7/2/0 | 9/0/0/0 | 0.125 | 0.004 |
| 6MWT, m | 360±138 | 420±51 | 462±55 | 0.208 | 0.050 |
| NT-proBNP, pg/ml | 538 (314−1089) | 204 (112−585) | 202 (116−392) | 0.156 | 0.652 |
| Hemodynamic characteristics | |||||
| Systolic PAP, mmHg | 69.3±19.3 | 55.1±21,3 | 43.4±14.6 | 0.028 | 0.144 |
| Diastolic PAP, mmHg | 27.3±8.3 | 22.9±5.3 | 16.6±5.3 | 0.054 | 0.062 |
| mPAP, mmHg | 43.4±11.3 | 34.8±12.5 | 26.0±6.4 | 0.018 | 0.082 |
| Mean PAD, mmHg | 7.6±4.4 | 5.0±3.0 | 6.8±2.6 | 0.196 | 0.191 |
| PCWP, mmHg | 10.0±3.6 | 9.1±2.1 | 11.7±4.4 | 0.426 | 0.052 |
| PVR, WU | 10.2±4.5 | 5.3±3.4 | 3.1±1.5 | 0.001 | 0.056 |
| Cardiac output, l/min | 4.5±1.4 | 5.0±1.2 | 5.0±1.4 | 0.128 | 0.849 |
| Cardiac index, l/min/m2 | 2.2±0.6 | 2.7±0.6 | 3.0±1.0 | 0.041 | 0.501 |
| SvO2, % | 63.7±9.8 | 65.4±9.0 | 70.5±5.0 | 0.631 | 0.087 |
| Absolute change in mPAP, mmHg | −8.7±8.7a | −8.8±13.3b | 0.985 | ||
| Absolute change in PVR, WU | −4.9±2.7a | −2.2±3.0b | 0.051 |
6MWT: 6-min walk test; BPA: pulmonary balloon angioplasty; mPAP: mean pulmonary artery pressure; PAP: pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation; VTE: venous thromboembolism; WHO: World Heath Organization.
The median time between first and last BPA sessions was four (4–5) months. Six-month follow-up was complete in nine of the 11 patients, and these nine were included in the efficacy analysis. One 82-year-old patient was excluded because she refused to continue the program after the second session resulted in non-major complications (included in the safety analysis), and another was excluded after finishing the program because she had not completed the six-month follow-up by the time the study ended.
In the nine patients who completed the BPA program and follow-up, the mean number of sessions was 5.8±1.6 (median 5.0), and the number of segments and vessels treated per patient in all sessions was 10.4±2.1 and 22.6±7.9, respectively. Clinical, laboratory and hemodynamic results at six months are displayed in Table 3 and Figure 3.
All patients presented statistically significant improvements in functional capacity as assessed both subjectively and objectively. The subjective assessment, as determined by WHO functional class, was translated into the absence of limitation in physical activity (class I) at six months after the last BPA session in all patients (0 vs. 100% of patients in class I, p=0.004). Objective improvement, as demonstrated by the 6MWT, was reflected in a mean increase of 42 m in distance covered (420±51 vs. 462±55 m, p=0.050). Oxygen therapy and prostaglandin analogs were also discontinued in 100% of previously medicated patients. However, there was no statistically significant change in serum NT-proBNP levels. No deaths were recorded during follow-up.
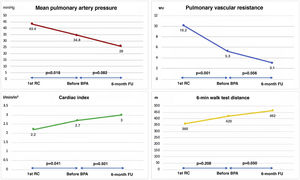
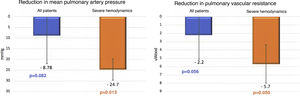
Regarding hemodynamic results, there were reductions of 25% in mPAP (34.8±12.5 vs. 26.0±6.4 mmHg, p=0.082) and of 42% in PVR (5.3±3.4 vs. 3.1±1.5 WU, p=0.056), which while showing a trend did not reach statistical significance. No significant difference was seen in cardiac index (2.7±0.6 vs. 3.0±1.0 l/min/m2, p=0.501). However, when patients with severely impaired hemodynamics (mPAP >40 mmHg) at the beginning of the BPA program were analyzed separately (Figure 4), there were statistically significant improvements in mPAP (50.3±0.6 vs. 24.7±5.5 mmHg, p=0.013) and in PVR (8.5±3.6 vs. 2.8±1.3 WU, p=0.050).
Comparison of changes in mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR) from the first session of balloon pulmonary angioplasty (BPA) to six-month follow-up between all patients undergoing the procedure (n=9) and those with severely impaired hemodynamics (mPAP >40 mmHg) at the beginning of the BPA program (n=3). A greater and statistically significant reduction in mPAP and PVR is seen in patients with severely impaired hemodynamics at baseline.
In the present study, we report our center’s initial experience as well as the first national data on treatment of CTEPH by BPA. Hospital Garcia de Orta became a Portuguese reference center for pulmonary hypertension in 2014, and began offering BPA in December 2017 under the technical and scientific guidance of experts from high-volume centers in France and Japan. In all cases, eligibility for BPA was decided by a multidisciplinary team, as recommended by the current guidelines.12
Our results confirm the efficacy of the technique in the treatment of CTEPH patients who are inoperable or who have residual or recurrent PH following surgery. Under optimal pulmonary vasodilator therapy, BPA provided additional improvements in functional class and exercise capacity that were significant in the short term. All patients were without limitations in physical activity at six-month follow-up, as shown by a mean increase of 42 m in 6MWT distance. Oxygen therapy and prostaglandin analogs were also discontinued in 100% of patients, resulting in improved quality of life and reductions in the costs of these therapies. Overall, there was a tendency toward improvements in hemodynamic parameters (mPAP and PVR). The reduction in mPAP in our study (25%) was similar to that recently reported in the French series29 (26%) and greater than in a German series28 (18%), but less than in the Japanese registry,23 in which the mean reduction was 44%. Regarding PVR, a reduction of 42% was seen in our study, comparable to that of the French registry29 (43%), greater than in the German registry28 and less than the 66% in the Japanese registry.23
There are several possible reasons for the differences between our results and those of the multicenter Japanese registry. The Japanese centers have considerably more experience and larger sample sizes, with 308 patients treated in their multicenter registry23 (unlike the French series,29 in which, although the sample was larger than ours, with 154 patients, the hemodynamic results were similar). There were differences in study populations, such as a greater incidence of symptomatic acute venous thromboembolism in our study (73% in our series and the French series29 compared to 35% in the Japanese registry23). There may be a more inflammatory thrombotic phenotype in European CTEPH patients than in Japan, which could lead to different responses to treatment.34 In addition, selection procedures for BPA could differ, since Japanese centers have less surgical experience and thus many patients undergoing BPA in Japan would be considered operable in European centers,35 unlike the majority of patients in our series who were inoperable or post-surgical. There was also a longer interval between CTEPH diagnosis and the first BPA session (median 55 months in our study), which meant that some patients could have developed peripheral pulmonary arterial disease, affecting the hemodynamic results of the BPA sessions. Furthermore, a larger proportion of our patients (82%) were under pulmonary vasodilator therapy at the beginning of the BPA program than in the Japanese series (72%)23 and in the French series (62%),29 and so the statistically significant improvements in hemodynamic variables between diagnosis and beginning the program due to vasodilator therapy may have reduced the absolute benefit observed with BPA.
The hemodynamic results in our study were better than in the German series, which may be explained by the number of vessels treated; although the median number of sessions per patient was similar, a mean of 3.9 vessels were treated in our center compared to 2.0 in the German series.28 As pointed out by Matsubara and Ogawa,36 to improve the efficacy of BPA, it is necessary to increase the number of vessels treated (by increasing the number of sessions per patient or the number of vessels treated per procedure). These authors also underline the importance of approaching difficult lesions, such as occlusions, despite the greater risk of complications, since the hemodynamic benefit from treating these obstructive lesions is potentially greater than from dilating less stenotic vessels. By avoiding treating highly complex lesions with a greater risk of complications (only 8.6% of treated lesions were total occlusions, whereas webs were dilated in most cases), the more conservative approach in the present series may also have contributed to the smaller hemodynamic improvement compared to the Japanese series.
Compared to the previously available therapies, the hemodynamic effects of BPA seen in our study were overall inferior to those reported for PEA surgery, which reduces mPAP by around 40% and PVR by around 60%.6,8,9,11 Nevertheless, in the subgroup of patients with severely impaired hemodynamics the hemodynamic benefits were similar to those of PEA, with reductions of 51% in mPAP and 67% in PVR, with final values within the normal range and thus with the potential to achieve hemodynamic normalization. These findings raise the question of the best way to measure the efficacy of BPA in patients with less severely impaired or even normal hemodynamics. In patients with less severely impaired hemodynamics, the efficacy of BPA should probably not be measured in terms of hemodynamics at rest, but rather by exercise parameters such as cardiopulmonary exercise testing or even exercise right heart catheterization.37
Regarding medical therapy, the profile of hemodynamic changes seen with BPA was different from that reported with pulmonary vasodilator therapy, since we observed improvements in mPAP and PVR but only very slight changes in cardiac index. Medical therapy, by contrast, tends mainly to improve cardiac output, with only modest effects on mPAP.15–17 The increase in cardiac output seen with pulmonary vasodilator therapy may be in part the result of systemic vasodilation.38 In the present series, cardiac index was normal (mean 2.7 l/min/m2) immediately before the beginning of the BPA program, so it would be hard to demonstrate any improvement in this parameter.
The procedure-related complication rate in our study was 25%, which is higher than reported in previous European series (9% in the German series28 and 11% in the French registry29), but lower than the 36% in the Japanese registry.23 However, these complications were mild, not requiring intensive care or leading to death. By contrast, the rate of fatal complications ranged from 2% to 4% in the three studies referred to above.23,28,29 Similarly to the German series, and in agreement with the literature,39–41 the most frequent complication was pulmonary vascular injury with or without pulmonary hemorrhage; the incidence of reperfusion pulmonary injury, the most common complication in the Japanese23 and French series,29 was low in our study.
There are several possible explanations for the high number of minor complications and low number of major complications, including reperfusion injury, in our study. These include the fact that all minor complications were recorded, and that the sessions were planned on the basis of thoracic computed tomography angiography studies that are known to provide more detailed information on the peripheral pulmonary arteries, enabling a more reliable assessment of vessel accessibility, morphology and diameter and lesion characteristics, and more accurate sizing of the angioplasty balloons.42,43 We did not use low molecular weight heparin bridging in patients taking direct oral anticoagulants, which is associated with a higher risk of bleeding.44 Furthermore, almost all of our patients were under optimal pulmonary vasodilator therapy before the first BPA session. The BPA procedure was guided by pressure wire in the first sessions in all patients with severely impaired hemodynamics, since higher mPAP and PVR are predictors of pulmonary injury.29 One of the main benefits of using a pressure wire is that pressures distal to the target lesions can be measured; another is that it helps to avoid overdilation of the target vessel. This may help to reduce angiographic complications, decreasing the risk of vascular injury during the procedure and of associated reperfusion pulmonary injury.45,46 Last but not least, we began our BPA program with improved techniques22 and based on the experience of experts in high-volume centers.29 As has been pointed out, there is a learning curve associated with the BPA technique, and its efficacy and safety improve with experience.29,35,36
Our study has some limitations. Firstly, it is a single-center study that includes a limited number of patients and BPA sessions. Secondly, a high proportion (82%) of our patients were under optimal therapy with at least one pulmonary vasodilator before the first BPA session, which could mean that medical therapy affected post-procedure results, as reported in previous studies.22,23,47 Finally, long-term follow-up results, required to assess the efficacy of the technique beyond six months post-procedure, are as yet unavailable.
ConclusionThe data presented here are of the first series of CTEPH patients treated by BPA in Portugal so far described in the literature. Before the implementation of the BPA program in our center, the only therapeutic alternatives for patients with inoperable CTEPH or residual or recurrent PH after surgery was medical therapy with pulmonary vasodilators. The implementation of our BPA program provided another therapeutic strategy for these patients. After the operationalization of this multidisciplinary program, its results need to be validated in terms of both efficacy and safety. The results described above confirm the significant benefits of the procedure in terms of pulmonary vascular hemodynamics, as well as of symptoms and functional capacity, which were sustained for at least six months of follow-up. These benefits were accompanied by a significant number of minor periprocedural complications, but there were no major procedure-related complications or deaths. Finally, the short- and long-term results of BPA need to be properly assessed in a prospective multicenter study.
FundingThe study has no funding to declare.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Calé R, Ferreira F, Pereira AR, Repolho D, Sebaiti D, Alegria S, et al., Segurança e eficácia da angioplastia pulmonar por balão em Portugal num centro de referência em hipertensão pulmonar, Rev Port Cardiol. 2021;40:727–737.