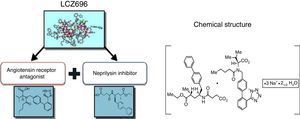
Sacubitril/valsartan (LCZ696), a supramolecular sodium salt complex of the neprilysin inhibitor prodrug sacubitril and the angiotensin receptor blocker (ARB) valsartan, was recently approved in the EU and the USA for the treatment of chronic heart failure (HF) with reduced ejection fraction (HFrEF) (NYHA class II-IV). Inhibition of chronically activated neurohormonal pathways (the renin-angiotensin-aldosterone system [RAAS] and sympathetic nervous system [SNS]) is central to the treatment of chronic HFrEF. Furthermore, enhancement of the natriuretic peptide (NP) system, with favorable cardiovascular (CV) and renal effects in HF, is a desirable therapeutic goal to complement RAAS and SNS blockade. Sacubitril/valsartan represents a novel pharmacological approach that acts by enhancing the NP system via inhibition of neprilysin (an enzyme that degrades NPs) and by suppressing the RAAS via AT1 receptor blockade, thereby producing more effective neurohormonal modulation than can be achieved with RAAS inhibition alone. In the large, randomized, double-blind PARADIGM-HF trial, replacement of an angiotensin-converting enzyme inhibitor (ACEI) (enalapril) with sacubitril/valsartan resulted in a significant improvement in morbidity and mortality in patients with HFrEF. Sacubitril/valsartan was superior to enalapril in reducing the risk of CV death or HF hospitalization (composite primary endpoint) and all-cause death, and in limiting progression of HF. Sacubitril/valsartan was generally well tolerated, with a comparable safety profile to enalapril; symptomatic hypotension was more common with sacubitril/valsartan, whereas renal dysfunction, hyperkalemia and cough were less common compared with enalapril. In summary, sacubitril/valsartan is a superior alternative to ACEIs/ARBs in the treatment of HFrEF, a recommendation that is reflected in many HF guidelines.
O sacubitril/valsartan (LCZ696) é um complexo supramolecular de sal de sódio do pró-fármaco sacubitril, inibidor da neprilisina, e do valsartan, um antagonista dos recetores da angiotensina (ARB), administrado por via oral, recentemente aprovado no tratamento da insuficiência crónica (classes II-IV NYHA) com fração de ejeção reduzida (IC-FER). A inibição neuro-hormonal é um elemento central no tratamento da insuficiência cardíaca. No entanto, os benefícios obtidos com o aumento de outros sistemas, tais como os péptidos natriuréticos, foram muito parcelares (e inconsistentes). O desenvolvimento do sacubitril/valsartan e os resultados do PARADIGM-HF mudaram a situação e abriram uma alternativa no tratamento da insuficiência cardíaca crónica. O PARADIGM-HF foi um grande estudo prospetivo aleatorizado, com dupla ocultação, que demonstrou que a morbimortalidade na insuficiência cardíaca pode ser melhorada com o sacubitril/valsartan, com redução significativa da morte cardiovascular ou da hospitalização por agravamento da insuficiência cardíaca. O sacubitril/valsartan foi também superior ao enalapril na redução da mortalidade total e na limitação da progressão da insuficiência cardíaca. O novo ARNi foi geralmente bem tolerado, sem incremento dos eventos adversos potencialmente fatais (apesar da maior incidência de hipotensão sintomática, mas com uma baixa ocorrência de angioedema). Por essa razão, o sacubitril/valsartan deve substituir o ACEi (ou o ARB) no tratamento da IC-FER, afirmação já expressa em muitas das novas recomendações terapêuticas.
Advances in the prevention, diagnosis and treatment of cardiovascular (CV) disease in recent years have led to significant reductions in age-adjusted CV mortality rates. Adjusted mortality due to diseases of the circulatory system in mainland Portugal fell by 15.5% between 2008 and 2012, reaching 144.7 per 100000 population in 2013.1
However, heart failure (HF) is an exception to this trend. Depending on the definition used, the prevalence of HF is 1-2% in the adult population, rising to ≥10% in those aged >70 years. At age 55, the lifetime risk of HF is 33% in men and 28% in women, and higher in hypertensives.2,3 The condition thus mainly affects the elderly and is a frequent cause of hospitalization. In the USA, HF is responsible for over a million hospital admissions a year.2 Although data are scarce, the prevalence of HF in Portugal has been estimated to be 4.4%, with similar figures for men (4.33%) and women (4.38%). Incidence rises with age, with the greatest disease burden in those aged over 65; in Portugal its prevalence reaches 12.67% in the 70-79 age-group and exceeds 16% in those aged over 80.4 There is little information on HF hospitalizations in this country, but they are also likely to increase significantly with age.5
Survival following a diagnosis of HF has improved considerably in the last 30 years, and age-adjusted mortality has also fallen.6 However, there is a paradox6: the prevalence of HF continues to increase, despite significant improvements in the prognosis of the individual factors that contribute to it, such as acute coronary syndrome, hypertension, valve disease and congenital heart disease. This disparity may be linked to the morbidity associated with these factors, even though mortality has fallen. Furthermore, longer life expectancy aggravates the effects of advanced age, such as increased cell death and/or apoptosis in myocytes and the adverse cardiac repercussions of comorbidities such as hypertension, type 2 diabetes, chronic kidney disease, chronic obstructive pulmonary disease and arrhythmias, especially atrial fibrillation (AF). Improvements in the prognosis of HF patients, albeit slow, also inevitably lead to a rise in the prevalence of the syndrome.6
In light of the varying but constant interaction throughout the natural history of the disease between cardiac function and the neurohormonal and inflammatory systems, current approaches to HF with reduced ejection fraction (HFrEF) are centered on blocking the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS), using angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) when ACEIs are not tolerated, beta-adrenergic blockers (BBs) and mineralocorticoid receptor antagonists (MRAs).3,7–9 However, even under optimized medical therapy, five-year mortality remains around 50%.5 It is thus essential to identify individuals at risk of developing HF (such as those with high body mass index or abdominal obesity, high fasting blood glucose, elevated systolic blood pressure (SBP), dyslipidemia and abnormal apolipoprotein B/apolipoprotein A-I ratio, and smokers) and to develop new drugs to improve the prognosis of the syndrome.
Simply put, HF is a clinical syndrome in which a series of local and systemic adaptations attempt to maintain CV homeostasis but in fact dysregulate it. This complex pathophysiological duality poses considerable challenges for attempts to determine and develop appropriate therapeutic strategies, which may not be mutually exclusive and should often be individually tailored. The multiple and inter-related processes involved in the progression and maintenance of HF are thus potential targets for therapeutic intervention, which should be complementary and ideally additive, with the aim of relieving symptoms, improving quality of life and reducing associated morbidity and mortality.
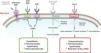
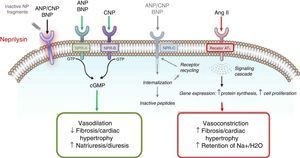
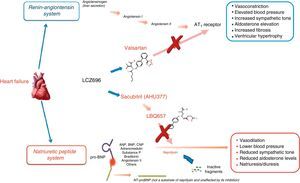
Understanding the role of the natriuretic peptide (NP) system, as well as that of the RAAS, in the regulation of blood pressure and fluid volume in both normal activity and pathological states (particularly HF) is a classic example of multidisciplinary collaboration that recently culminated in the development and introduction of the first angiotensin receptor/neprilysin inhibitor (ARNI),10,11 sacubitril/valsartan (LCZ696) (Figure 1). Neprilysin (NEP), also known as neutral endopeptidase, CD10. enkephalinase, common acute lymphoblastic leukemia antigen and endopeptidase 24.11, is an enzyme that cleaves NPs (Figure 2). Its main effects and characteristics are summarized in Table 1.18
Mechanisms of action of neprilysin and natriuretic peptides, in parallel with the renin-angiotensin-aldosterone system (adapted from Bayés-Genís et al.13).
Overview of the natriuretic peptide system.14–19
| Natriuretic peptide | ANP | BNP | CNP |
| Location | Atria | Ventricles | Vascular endothelial cells |
| Triggers | Atrial distension | Increased ventricular volume | Increased shear stress |
| Receptor | NPR-A | NPR-A | NPR-B |
| Physiological actions | Natriuresis and diuresis Vasodilation RAAS and SNS suppression Increased renal blood flow and GFR Increased myocardial relaxation Lipid mobilization, metabolic effects Anti-hypertrophic Antifibrotic Increased endothelial permeability Anti-inflammatory | Natriuresis and diuresis Vasodilation RAAS and SNS suppression Increased renal blood flow and GFR Increased myocardial relaxation Lipid mobilization, metabolic effects Antifibrotic | Vasodilation Anti-hypertrophic Antifibrotic Anti-inflammatory Antithrombotic Bone growth regulation |
| Clearance of NP/enzymatic degradation | Clearance via NPR-C | Clearance via NPR-C | Clearance via NPR-C |
| NEP degradation | NEP degradation | NEP degradation |
ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; CNP: C-type natriuretic peptide; GFR: glomerular filtration rate; NEP: neprilysin; NPR-A: type A natriuretic peptide receptor; NPR-B: type B natriuretic peptide receptor; NPR-C: type C natriuretic peptide receptor; RAAS: renin-angiotensin-aldosterone system; SNS: sympathetic nervous system.
Attempts over the years to achieve effective NEP inhibition have faced obstacles in understanding the pathophysiological and functional mechanisms involved, as well as uncertainties regarding dosages, methods of administration, and therapeutic combinations, frequently leading to blind alleys and misunderstandings, but progress has been made.2,10–13,20,21
The first synthetic NEP inhibitor was thiorfan, the active metabolite of racecadotril. In healthy volunteers as well as in patients with hypertension and HF, NEP inhibitors increase plasma concentrations of endogenous atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) and circulating and urinary cGMP levels, and lead to short-term hemodynamic improvement, with a slight and transient hypotensive effect13,22–24 but without significant impact on severe HF. Investigation of pharmacological NEP inhibition continued with studies on candoxatril followed by ecadotril, but these were not shown to be effective and research efforts have stalled. Attempts to develop NEP inhibitors monotherapy were replaced by combinations with ACEIs (vasopeptidase inhibitors), including omapatrilat (in the IMPRESS and OVERTURE trials), fasidotril, sampatrilat and mixanpril.13 However, the observed benefits were overshadowed by the risk of severe angioedema25 caused by excess bradykinin, des-Arg9-bradykinin, and possibly substance P,22,26 which effectively ended the development of vasopeptidase inhibitors. Inhibition of the three enzymes involved in bradykinin degradation, in descending order of importance angiotensin-converting enzyme, aminopeptidase P and NEP,22 leads to accumulation of bradykinins in sufficient quantities to cause angioedema, which is potentially fatal if it affects the upper airway.
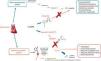
Awareness of this risk and of the difficulty in determining the appropriate dose for HF (which was not achieved in the case of omapatrilat) prompted the next step, combining an ARB and an NEP inhibitor, to be taken twice daily. The result was the first ARNI, sacubitril/valsartan (LCZ696)24,27 (Figure 3). The physiological effects of different drugs that modulate NEP and the RAAS are listed in Table 2.13
Mechanism of action of LCZ696 (from Vardeny et al.25).
Physiological effects of isolated inhibition of neprilysin and angiotensin-converting enzyme and combined inhibition of neprilysin and the renin-angiotensin-aldosterone system (adapted from Bayes-Genis et al.13).
| NEPI | ACEI | NEPI + ACEI | ARNI | |
|---|---|---|---|---|
| Effects on plasma natriuretic peptide levels | ||||
| Angiotensin II | ↑ | ↓ | ↓ | ↔ ↓ |
| Renin | ↓ | ↔ | ↔ ↑ | ↑ |
| Aldosterone | ↓ | ↔ | ↓ | ↓ |
| NPs or cGMP | ↑ | ↔ | ↑ | ↑ |
| ET-1 | ↑ | ↔ | ↑ | ↓ |
| Big ET-1 | ↑ | ↑ | ||
| Bradykinin | ↑ | ↑ | ↑↑ | ↑ |
| Physiological effects | ||||
| Blood pressure | ↔ | ↓ | ↓ | |
| Sodium excretion | ↑ | ↑ | ↑ | ↑↑ |
| Cardiac hypertrophy | ↔ ↓ | ↓ | ↓↓ | ↓↓ |
| CV fibrosis | ↓ | ↓ | ↓↓ | ↓↓ |
↑: increased; ↑↑: significantly increased; ↓: decreased; ↓↓: significantly decreased; ↔; unchanged; ACEI: angiotensin-converting enzyme inhibitor; ARNI: angiotensin receptor/neprilysin inhibitor; CV: cardiovascular; cGMP: cyclic guanosine monophosphate; ET-1: endothelin 1; NEPI: neprilysin inhibitor; NPs: natriuretic peptides.
Despite what its name suggests, sacubitril/valsartan is not a fixed combination of drugs but a supramolecular sodium salt complex of the NEP inhibitor prodrug sacubitril (AHU377) and the diprotic ARB valsartan in a 1:1 molecular ratio.23,24,27–30 It has a stable crystalline structure and is highly water-soluble, and contains six anionic molecules of sacubitril and six of valsartan complexed with sodium cations and water molecules. After ingestion, valsartan/sacubitril dissociates to valsartan and sacubitril. The target maintenance dose in HFrEF is one 200 mg tablet, containing 97 mg sacubitril and 103 mg valsartan, twice daily.
Sacubitril, the prodrug, is metabolized in vivo by enzymatic cleavage of the ethyl ester to sacubitrilat (LBQ657), the active metabolite, which inhibits NEP without inhibiting aminopeptidase P. Valsartan inhibits the RAAS by binding to and blocking the AT1 angiotensin II receptor.21,23–25
In pharmacodynamic terms, sacubitril/valsartan causes a non-sustained increase in natriuresis and urinary cGMP (which is used as a biomarker for NEP inhibition) and a decrease in plasma mid-regional pro-ANP (MR-proANP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP).23 These changes are seen consistently, with increased urinary ANP levels and urinary and plasma cGMP and lower plasma levels of NT-proBNP, aldosterone and ET-1 (Figure 3). In a dose escalation study in healthy participants there was a maximal 40% increase in mean cGMP levels at 4 h and significant increases at 12 h post-dose, with return to baseline levels at 24 h after administration of LCZ696.23,31 In the same study, significant dose-dependent effects of the valsartan moiety led to increases in renin concentration, plasma renin activity and angiotensin II, which were still observed 24 h after dosing. This also implies that BNP is not a viable biomarker in HF patients treated with sacubitril/valsartan, whereas NT-proBNP remains so, since it is not a substrate for NEP.
PharmacokineticsTable 3 summarizes the main pharmacokinetic properties of sacubitril/valsartan.2 Following oral administration, the complex is rapidly absorbed (food intake has no clinically significant impact) and, as described above, dissociates into valsartan and sacubitril. The latter is then metabolized by carboxylesterases 1b and 1c to the active metabolite, sacubitrilat. These three molecules reach their maximum plasma concentration in 90, 3-60, and 120 min, respectively. The absolute oral bioavailability of sacubitril is over 60% and that of valsartan is around 23%.2,23,24,29,32 The valsartan salt used in the complex differs from that used in monotherapy, the plasma bioavailability following 400 mg of LCZ696 being equivalent to 320 mg of valsartan, corresponding to a 40% higher systemic exposure to valsartan (possibly related to its presence in the ionic form rather than the free acid form in sacubitril/valsartan).33 A dose of 97/103 mg of sacubitril/valsartan is thus bioequivalent to 160 mg of valsartan.
Summary of the pharmacokinetics of sacubitril/valsartan (adapted from Mills and Vardeny2).
| Valsartan | Sacubitril (AHU377) | Sacubitrilat (LBQ657) | |
|---|---|---|---|
| Bioavailability (%) | 23 | >60 | |
| Volume of distribution (l) | 75 | 103 | |
| Protein binding (%) | 94-97 | ||
| Tmax (h) | 1.5 | 0.5 | 2 |
| Metabolism | Minimal (≈20%) (<10% as hydroxyl metabolite) | Metabolized to sacubitrilat by esterases | No significant metabolism |
| Excretion | Renal (≈13%) Feces (86%) | Renal (52-68%), primarily as sacubitrilat | Feces (37-48%). primarily as sacubitrilat |
| Half life (h) | 9.9 | 1.4 | 11.5 |
Half life: plasma elimination half-life; Tmax: time to maximum plasma concentration.
Following twice daily dosing, steady-state levels are reached in three days. At steady state, sacubitril and valsartan do not accumulate significantly, while the active metabolite accumulates 1.6-fold. Sacubitril, sacubitrilat and valsartan are highly bound to plasma proteins (94-97%), with a mean apparent volume of distribution of valsartan and sacubitril of 75 l and 103 l, respectively. Sacubitrilat crosses the blood-brain barrier to a limited extent (exposure in cerebrospinal fluid is 0.28% of plasma level).23,28
Following bioactivation of the prodrug, sacubitrilat undergoes no further biotransformation. Valsartan is partially metabolized (≈20%), presumably by CYP2C9, mainly to a hydroxyl metabolite, valeryl-4-hydroxy valsartan, found in low concentrations (<10%) in plasma. About 52-68% of sacubitril (mainly as sacubitrilat) and 13% of valsartan are excreted in urine, while 37-48% of sacubitril (mainly as sacubitrilat) and 86% of valsartan are excreted in feces. The elimination half-life of sacubitril, sacubitrilat and valsartan is 1.43 h, 11.48 h and 9.90 h, respectively.23,24,28,32
The pharmacokinetics of sacubitril/valsartan is unaffected by gender, but in the elderly exposure to sacubitrilat and valsartan increases by 42% and 30%, respectively, presumably due to renal and/or liver dysfunction. Unlike valsartan, systemic exposure to sacubitrilat is increased in patients with mild to severe renal dysfunction, doubling with glomerular filtration rate (GFR) 15 ml/min/1.73 m2 compared to 30 ml/min/1.73 m2. Similarly, systemic exposure to sacubitril, sacubitrilat and valsartan is increased in patients with mild to moderate liver dysfunction.23,24,28
Drug interactionsSacubitril inhibits organic anion-transporting polypeptides (OATP), particularly OATP1B1 and OATP1B3, which are expressed in the basolateral membrane of hepatocytes and are involved in clearance by the liver of various drugs including statins and furosemide. Co-administration with sacubitril/valsartan increases systemic exposure to atorvastatin and its metabolites. Caution should therefore be exercised in the concomitant use with HMG-CoA reductase inhibitors.13,28
Concomitant use of furosemide results in reductions in its maximum concentration and area under the curve (AUC) of 50% and 28%, respectively,24,27 and a decrease in urinary sodium excretion. Sacubitril/valsartan also reduces systemic exposure to hydrochlorothiazide and metformin (the latter by 23%). The clinical relevance of these findings is unknown.13,28 In the elderly, patients with volume depletion and those with impaired renal function, concomitant use of sacubitril/valsartan and non-steroidal anti-inflammatory drugs may worsen renal dysfunction, and this should be carefully monitored.
Sacubitril/valsartan in the treatment of heart failureHeart failure with reduced ejection fractionSacubitril/valsartan has been approved and “indicated in adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction” by the European Medicines Agency (EMA) since November 2015.28,34 Approval was based on the results of the Prospective Comparison of ARNI With ACEI to Determine Impact in Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial.35
The double-blind PARADIGM-HF trial compared sacubitril/valsartan 200 mg twice daily with enalapril 10 mg twice daily in patients with HFrEF. The primary outcome was a composite of death from cardiovascular causes or hospitalization for HF. Included patients were being followed as outpatients and had been diagnosed with established chronic HF, in New York Heart Association (NYHA) class II-IV and left ventricular ejection fraction (LVEF) ≤40% (changed to ≤35% because 961 randomized patients had LVEF >35%), with BNP ≥150 pg/ml (NT-proBNP ≥600 pg/ml) or BNP ≥100 pg/ml (NT-proBNP ≥400 pg/ml) if they had been hospitalized for HF within the previous 12 months. Overall, 4.6% were in NYHA class I, 70.5% in class II, 24% in class III and only 0.7% in class IV. The mean age of the study population was 64 years and 87% were male, mostly white (66%) or Asian (18%). Patients were required to be taking a stable dose of an ACE inhibitor (or ARB) equivalent to at least 10 mg of enalapril daily for at least four weeks before screening. They were also required to be taking a stable dose of a beta-blocker (unless contraindicated or not tolerated) for at least four weeks before screening. There was a run-in period for enalapril followed by another for sacubitril/valsartan (with mean drug exposure time of 15 and 29 days, respectively), to confirm that study participants tolerated both drugs, before the randomized double-blind treatment stage.
The trial was stopped early, after a median follow-up of 27 months, because pre-specified criteria were attained for a compelling difference between groups: in the three interim efficacy analyses, the last when two-thirds of the primary events had occurred, a highly significant reduction was seen for both death from CV causes and the primary endpoint.36,37 Other treatment followed international guidelines, with 82% of patients receiving diuretics, over 90% beta-blockers, over 50% MRAs, and 30% digoxin; 15% had an implantable cardioverter-defibrillator (ICD) and 7% a cardiac resynchronization therapy (CRT) device at randomization.38 At the last assessment, the mean dose of sacubitril/valsartan was 375 mg and that of enalapril was 18.9 mg.23
Sacubitril/valsartan was significantly superior to enalapril, reducing the risk of the primary endpoint by 20% (hazard ratio [HR]: 0.80; 95% confidence interval [CI]: 0.73-0.87; p<0.0001), as well as its individual components, reducing CV death by 20% (HR: 0.80; 95% CI: 0.71-0.89; p<0.0001) and hospitalization for HF by 21% (HR: 0.79; 95% CI: 0.71-0.89; p<0.0001). The reduction in CV death was mainly due to reduced risk of sudden death (HR: 0.80; 95% CI: 0.68-0.94; p=0.008) and death due to worsening HF (HR: 0.79; 95% CI: 0.64-0.98; p=0.034).39 The number needed to treat (NNT) over 27 months to avoid one CV death or hospitalization due to HF was 21, while the NNT to avoid one CD death was 32 and to avoid one hospitalization due to HF was 36.34 The reductions in CV death and in the primary endpoint were consistent in all subgroups35 and were independent of age, LVEF, SBP, baseline risk according to the MAGGIC score, and glycated hemoglobin level.23
Of the secondary endpoints, sacubitril/valsartan reduced all-cause death by 16% (HR: 0.84; 95% CI: 0.76-0.93; p=0.0005), with an estimated increase in life expectancy of 1.3 years in participants aged 65 (life expectancy was 10 years in those treated with enalapril and 11.4 years in those taking sacubitril/valsartan). Significantly, sudden cardiac death was also reduced by treatment with sacubitril/valsartan compared with enalapril (HR 0.80; p=0.008).39 The ARNI also significantly improved symptoms and physical limitations as measured by both the Kansas City Cardiomyopathy Questionnaire and NYHA functional class.40 There were no differences between the treatments in risk for worsening renal function or new-onset AF. The use of health resources involved in treatment was less with sacubitril/valsartan, with significantly fewer total hospitalizations for heart failure (relative risk [RR]: 0.77; 95% CI: 0.67-0.89; p<0.001), hospitalizations for CV reason (RR: 0.84; 95% CI: 0.76-0.92; p<0.001), hospitalizations for any reason (RR: 0.84; 95% CI: 0.78-0.91; p<0.001), days in intensive care for any reason (RR: 0.79; 95% CI: 0.63-0.99; p=0.0434), and total number of emergency department visits for HF (RR: 0.70; 95% CI: 0.52-0.94; p=0.0170).40 The new drug also reduced the need for intensification of treatment (520 vs. 604 patients; HR 0.84; p=0.003), for intravenous positive inotropic agents (relative risk reduction [RRR] 31%, p<0.001) and for implantation of a heart failure device or cardiac transplantation (RRR 22%, p=0.07).40
The reduction in mortality obtained in this study was similar to that compared to placebo in the treatment arm of the Studies of Left Ventricular Dysfunction (SOLVD-T), which established ACEIs as a first-line treatment for HD.41 It should be noted that the dose of enalapril (10 mg twice daily) was higher than in SOLVD-T.
The PARADIGM-HF trial was notable for its prospective, randomized and controlled design, the suitability of its comparator, the importance and consistency of the risk reductions (it has been called “the 20% trial”42) (Table 4) and its statistical analysis and power to determine clinically relevant differences in well-defined endpoints.
PARADIGM-HF: summary of results for the combined primary endpoint and its components and total mortality.
| Endpoint | HR (95% CI) | RRR | p |
|---|---|---|---|
| Primary composite endpoint of CV death and hospitalization due to HF | 0.80 (0.73-0.87) | 20% | <0.001 |
| Individual components of the primary composite endpoint | |||
| CV death | 0.80 (0.71-0.89) | 20% | <0.001 |
| 1st hospitalization due to HF | 0.79 (0.71-0.89) | 21% | <0.001 |
| Secondary endpoint | |||
| All-cause mortality | 0.84 (0.76-0.93) | 16% | 0.0005 |
CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio.
Sacubitril/valsartan was associated with fewer serious adverse events and adverse events leading to discontinuation of the study (10.7% vs. 12.2%).35 Hyperkalemia (11.6% vs. 14%), cough (8.8% vs. 12.6%) and renal impairment (10.1% vs. 11.5%) were significantly less frequent in patients treated with sacubitril/valsartan. However, symptomatic hypotension was significantly more common in the sacubitril/valsartan group (14.0% vs. 9.2%), although it rarely led to discontinuation of the drug.35 Angioedema occurred in 29 patients in the sacubitril/valsartan arm and in 25 patients in the enalapril arm. In no cases was it considered life-threatening and no patient had airway compromise.35 A biomarker substudy confirmed that patients treated with sacubitril/valsartan had higher plasma BNP and urinary cGMP levels (as expected from NEP inhibition) but lower levels of NT-proBNP (reflecting reduced myocardial stress) and troponin (due to reduced myocardial injury). Because BNP is a substrate for NEP, levels of BNP reflect the action of the drug, whereas levels of NT-proBNP reflect its cardioprotective effect. This apparent discrepancy in the changes in these biomarkers with sacubitril/valsartan, contrasting with the parallel changes usually seen in the natural history of HF, is interesting and merits further attention.13,40
Heart failure with preserved ejection fractionHF with preserved ejection fraction (HFpEF) is a diagnostic and therapeutic challenge,3 since LVEF is normal (≥50%) and HF signs and symptoms are frequently non-specific and difficult to distinguish from other clinical conditions, which may also be present. Diagnosis in elderly patients with comorbidities and no obvious signs of central fluid overload is cumbersome, but should be supported by objective measures of cardiac dysfunction at rest or during exercise.3 The diagnosis of HFpEF requires the presence of symptoms and/or signs of HF, ‘preserved’ LVEF, elevated levels of NPs (BNP >35 pg/ml and/or NT-proBNP >125 pg/ml, and evidence of cardiac functional and structural alterations underlying HF.
There is to date no firm evidence that any drug alters the natural history of HFpEF,3,7 but sacubitril/valsartan is also intended to be used to treat it. The phase 2 trial Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) assessed the efficacy and safety of sacubitril/valsartan 200 mg twice daily vs. valsartan 160 mg twice daily in patients with HFpEF.43 Randomization was preceded by a two-week run-in with placebo. The trial included 301 patients aged over 40 years with a history of symptomatic HF and medicated with diuretics, LVEF ≥45%, NT-proBNP >400 pg/ml, GFR ≥30 ml/min/1.73 m2 and serum potassium ≤5.2 mmol/l, treated and followed for 36 weeks.43 Mean age was 71 years and 57% were women; 94% had hypertension, 38% diabetes, 42% previous HF hospitalization, 42% a history of AF and 21% previous myocardial infarction. Blood pressure was controlled in both arms (median 136/79 mmHg) and baseline medication was similar, with 93% of patients on ACEIs (54%)/ARBs (39%), 79% on beta-blockers and 21% on MRAs). The first echocardiographic assessment showed increased E/e’ ratio and increased indexed left atrial volume, consistent with slightly elevated left ventricular filling pressures.
The primary endpoint of the PARAMOUNT trial, change in NT-proBNP at 12 weeks, was achieved significantly more often in the sacubitril/valsartan group (p=0.005), mean NT-proBNP falling from 783 to 605 pg/ml compared to 862 to 835 pg/ml with valsartan. The difference between the groups decreased over 36 weeks (p=0.20). At 36 weeks, left atrial reverse remodeling was observed in the sacubitril/valsartan group, reflected in significant reductions in left atrial width (p=0.03) and indexed left atrial volume (p=0.007), which were not seen in the valsartan group and that were more marked in patients without AF.
Estimated GFR (eGFR) (unlike the albumin-to-creatinine ratio) declined significantly less in the sacubitril/valsartan group than in the valsartan group (-1.5 vs. -5.2 ml/min/1.73 m2; p=0.002), and worsening renal function, defined as serum creatinine increase of >0.3 mg/dl and/or >25% between two time-points, was less common in the sacubitril/valsartan group, although this difference was not statistically significant.44 Despite a more marked reduction in blood pressure with sacubitril/valsartan (a mean of 9 mmHg SBP and 5 mmHg diastolic blood pressure vs. 3 and 2 mmHg, respectively, with valsartan), there was poor correlation between change in SBP and change in NT-proBNP at 12 weeks and in GFR, NYHA functional class and left atrial volume.45 The target dose of sacubitril/valsartan and of valsartan was achieved in 81% and 78% of patients, respectively; more patients in the valsartan group required loop diuretics. Sacubitril/valsartan was well tolerated, and the incidence of serious adverse effects, hypotension, renal dysfunction and hyperkalemia did not differ between the groups; there was one case of angioedema in the sacubitril/valsartan group.43
Although the PARAMOUNT trial was not designed to analyze clinical endpoints, it indicated possible benefits of sacubitril/valsartan in HFpEF that may or may not be confirmed by future studies. One of these is the Prospective comparison of ARni with Arb Global Outcomes in heart failure with preserved ejectioN fraction (PARAGON-HF) trial, which aims to assess the effect of sacubitril/valsartan compared to valsartan in the reduction of CV death and HF hospitalization in patients with HFpEF (ClinicalTrials.gov identifier NCT01920711).46 Data collection began in July 2014 and is estimated to be complete in May 2019.
Safety profile and dosing of sacubitril/valsartanThe most frequent adverse effects of sacubitril/valsartan (hypotension, hyperkalemia and renal impairment), mentioned above, are well documented.23,24,26–28,35 However, some of them merit particular attention42,47 and are the basis of the risk management plan approved by the EMA34,48 (Table 5).
| Important potential risks | ||
|---|---|---|
| Risk | What is known | Recommendation |
| Hepatotoxicity | Limited liver metabolism, suggesting a low risk of liver injury | Caution in patients with moderate hepatic impairment (Child-Pugh B) or with AST/ALT twice UNL |
| Contraindicated in patients with severe hepatic impairment, biliary cirrhosis or cholestasis (Child-Pugh C) | ||
| Cognitive impairment | NEP is one of the proteases that degrade Aβ, a component of the amyloid plaques found in Alzheimer's diseasea | Assessment of cognitive function in PARAGON-HF |
| No evidence in monkeys treated with 300 mg/kg/day for 39 weeks | Comprehensive assessment of cognitive function and of brain amyloid plaque deposition by PET in assessment of patients with HFpEF | |
| No greater incidence of cognitive adverse effects or dementia49 in existing studies | ||
| In healthy volunteers, sacubitrilat reached concentrations sufficient to inhibit NEP in CSF, but did not cause changes in CSF levels of aggregable Aβ isoforms, despite increasing soluble Aβ 1-3851 | ||
| Statin drug-drug interaction | May increase plasma statin levels, leading to adverse effects | Caution when co-administered with statins |
| Post-authorization safety study assessing risk of statin-related events associated with concomitant use of sacubitril/valsartan | ||
| Angioedema | Laryngeal angioedema can be life-threatening | If angioedema occurs, sacubitril/valsartan should be immediately discontinued and appropriate treatment and monitoring should be provided until complete and sustained resolution of signs and symptoms has occurred |
| Incidence of angioedema: 0.5% (without airway compromise) vs. 0.2% with enalapril (bear in mind run-in period and possible selection bias) | Contraindicated in patients with a known history of angioedema related to previous ACEI or ARB therapy or with hereditary or idiopathic angioedema | |
| No studies on patients with history of angioedema | ||
| Higher risk in black individuals (only ≈5% of trial subjects) | ||
| Pregnancy and breast-feeding | No data on the use of sacubitril in pregnant women. Studies in animals have shown reproductive toxicity | Not recommended during the first trimester of pregnancy and contraindicated during the second and third trimesters |
| It is not known whether sacubitril/valsartan is excreted in human milk (but it is excreted in the milk of lactating rats) | Not recommended during breast-feeding due to potential risk to breast-fed newborns/infants | |
| No data on effects on human fertility | ||
| Missing information | ||
| Pediatric patients | PD/PK, safety and efficacy in children and adolescents aged below 18 years with HF have not been established | Not recommended for patients aged <18 years |
| Phase II/Phase III trial assessing PK/PD of single-dose sacubitril/valsartan, followed by a 52-week efficacy and safety study comparing sacubitril/valsartan with enalapril in children with HF | ||
| Patients with severe renal impairment | Very limited experience in patients with severe renal impairment (eGFR <30 ml/min/1.73 m2) | Assessment and monitoring of renal function |
| Greater risk of hypotension with renal impairment | No dose adjustment is required in patients with mild renal impairment (eGFR 60-90 ml/min/1.73 m2) | |
| No experience in patients with end-stage renal disease | Starting dose of 24 mg/26 mg twice daily recommended for patients with eGFR 30-60 ml/min/1.73 m2 | |
| May be associated with worsening renal function, which may necessitate dose titration | Should be used with caution in patients with eGFR <30 ml/min/1.73 m2, with starting dose 24 mg/26 mg twice daily | |
| Not recommended in end-stage renal disease | ||
| Significantly higher risk with dehydration or concomitant use of NSAIDs | ||
| ACEI/ARB naïve patients | Only a few patients who had not previously used ACEIs/ARBs were included in studies on sacubitril/valsartan | Initial dose of 24/26 mg twice daily and slowly titrated (every 3-4 weeks) |
| Safety and tolerability in these patients were similar | ||
| Patients in NYHA class IV (and other populations) | Limited clinical experience in patients in NYHA class IV (only 0.7% in PARADIGM-HF) | Caution should be exercised when initiating treatment in patients in NYHA class IV |
| No solid data on hospitalized patients and those with MI-related HF | Bear in mind the inclusion and exclusion criteria of PARADIGM-HF; biomarker criteria are not included in the dosing information from the EMA or FDA | |
| Dual RAAS blockade | Risk of angioedema in association with ACEI | Concomitant use with ACEI contraindicated |
| Effects of combination with aliskiren unknown | Must not be started until 36 h after last dose of ACEI dose (and vice-versa); a longer washout is desirable because ACEIs have a prolonged duration of action | |
| Supramolecular complex of NEP inhibitor and valsartan | Not recommended in association with direct renin inhibitors and combination with direct renin inhibitors is contraindicated in patients with diabetes or renal impairment | |
| Should not be co-administered with another ARB | ||
| Hypotension | Symptomatic hypotension is the most common adverse event in clinical trials, but without significant differences as a reason for discontinuation of therapy | Treatment should not be initiated unless SBP is ≥100 mmHg and blood pressure should be monitored at beginning of treatment or during dose adjustment |
| Especially in patients aged ≥65 years, with renal impairment or SBP <112 mmHg | If hypotension occurs, temporary down-titration or discontinuation is recommended, as well as dosing adjustment of diuretics and other antihypertensive treatment; dose adjustment of diuretics, concomitant antihypertensives and treatment of other causes of hypotension should be considered | |
| Sodium and/or volume depletion should be corrected before starting treatment, however, such corrective action must be weighed against the risk of volume overload | ||
| Changes in K+ | May be associated with increased risk of hyperkalemia | Treatment should not be initiated if K+ >5.4 mmol/l |
| Hypokalemia may also occur | Monitoring of K+ is recommended, especially in patients with risk factors such as renal impairment, diabetes or hypoaldosteronism or who are on a high potassium diet | |
| Concomitant use of potassium-sparing drugs increases risk of hyperkalemia | ||
Aβ: amyloid beta; ACEI: angiotensin-converting enzyme inhibitor; ALT: alanine aminotransferase; ARB: angiotensin receptor blocker; AST: aspartate aminotransferase; CSF: cerebrospinal fluid; eGFR: estimated glomerular filtration rate; EMA: European Medicines Agency; FDA: Food and Drug Administration; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; K+: serum potassium; MI: myocardial infarction; NEP: neprilysin; NSAIDs: non-steroidal anti-inflammatory drugs; PD: pharmacodynamics; PET: positron emission tomography; PK: pharmacokinetics; SBP: systolic blood pressure; UNL: upper normal limit.
Angiotensin-converting enzyme also degrades amyloid beta peptides, thus ACEIs can also theoretically promote amyloid beta accumulation.23
The usual recommended starting dose of sacubitril/valsartan is 49/51 mg twice daily. The dose should be doubled every 2-4 weeks until the target dose of 97/103 mg twice daily is reached, if tolerated.34,50 In patients with SBP ≤95 mmHg, symptomatic hypotension, hyperkalemia or renal impairment, adjustment of concomitant medication, or temporary down-titration or discontinuation of sacubitril/valsartan is recommended. In patients with SBP ≥100-110 mmHg (and in some elderly patients), a starting dose of 24/26 mg twice daily should be considered. Sacubitril/valsartan should not be administered to patients with serum potassium >5.4 mmol/l or with SBP <100 mmHg (Table 5). It should not be co-administered with an ACEI or an ARB. Due to the potential risk of angioedema, renal impairment and hyperkalemia, it must not be started for at least 36 h after discontinuing ACEI therapy.52
ConclusionClinical evidence supports the indications for sacubitril/valsartan approved by regulatory bodies.28,34,53 However, these indications do not appear to be limited to the inclusion and exclusion criteria of the PARADIGM-HF trial. Sacubitril/valsartan is an innovative drug that confirms and widens the principle of neurohormonal modulation in the treatment of HF and is a valuable addition to the best treatment available in symptomatic chronic HFrEF (although other drugs, including beta-blockers, MRAs, ivabradine and digoxin, and ICDs and CRT devices, should of course continue to be used in accordance with the guidelines).3,7–9Table 6 summarizes the indications in the international guidelines for the use of sacubitril/valsartan in the treatment of HFrEF.
Indications in the international guidelines for the use of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction.
| Entity | Indication | Reference |
|---|---|---|
| European Medicines Agency | … indicated in adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction | 28 |
| US Food and Drug Administration | … indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA class II-IV) and reduced ejection fraction. | 53 |
| … usually administered in conjunction with other heart failure therapies, in place of an ACE inhibitor or other ARB | ||
| UK National Institute for Health and Care Excellence | … recommended as an option for treating symptomatic chronic heart failure with reduced ejection fraction, only in people: | 54 |
| • with New York Heart Association (NYHA) class II to IV symptoms and • with a left ventricular ejection fraction of 35% or less and • who are already taking a stable dose of ACEIs or ARBs | ||
| … should be started by a heart failure specialist with access to a multidisciplinary heart failure team. Dose titration and monitoring should be performed by the most appropriate team member … | ||
| European Society of Cardiology and Heart Failure Association | … recommended as a replacement for an ACEI to further reduce the risk of HF hospitalization and death in ambulatory patients with HFrEF who remain symptomatic despite optimal treatment with an ACEI, a beta-blocker and an MRA (class I, level B) | 3 |
| American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America | The clinical strategy of inhibition of the RAAS with ACEIs (level of evidence: A) (9-14), or ARBs (level of evidence: A), or ARNI (level of evidence: B-R) in conjunction with beta blockers, and aldosterone antagonists in selected patients, is recommended for patients with chronic HFrEF to reduce morbidity and mortality (class 1) | 8 |
| In patients with chronic symptomatic HFrEF NYHA class II or III who tolerate an ACEI or ARB, replacement by an ARNI is recommended to further reduce morbidity and mortality (class 1) | ||
| Canadian Cardiovascular Society | … patients with mild to moderate HF, an EF <40%, an elevated NP level or hospitalization for HF in the past 12 months, a serum potassium <5.2 mmol/l, and an eGFR 30 ml/min and treated with appropriate doses of guideline-directed medical therapy should be treated with sacubitril/valsartan in place of an ACEI or an ARB (conditional recommendation; high quality evidence) | 9,55 |
| At end of titration of triple therapy for those with LVEF <40%, elevated NP levels may prompt switching ACEI (or ARB) therapy to sacubitril/valsartan |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor/neprilysin inhibitor; eGFR: estimated glomerular filtration rate; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; K+: serum potassium; LVEF: left ventricular ejection fraction; NP: natriuretic peptide; NYHA: New York Heart Association.
Morbidity and mortality associated with HFrEF – even in only mildly symptomatic patients – remain high. Five-year survival in HF is greater than in many cancers, and the economic, social and human costs are high. There is thus an ethical imperative to improve this situation, in terms of primary as well as secondary and tertiary prevention. The Portuguese National Program for Cerebro- and Cardiovascular Diseases, in partnership with the Foundation for Science and Technology, recently announced their support56 for the first Joint Transnational Call (JTC2016) for the European Research Area Network on Cardiovascular Diseases, which, among other aims, focuses on early recognition and prognosis in HD, as well as innovative approaches to prevention and treatment, including reversal of cardiac remodeling. Now is thus a good time to reflect on the results of the PARADIGM-HF trial and to incorporate them into clinical practice. The ease and speed with which sacubitril/valsartan is incorporated into the therapeutic arsenal for HFrEF (and, in the future, possibly also HFpEF) depend on various factors, which will include unbiased and thorough cost-effective analyses. This review article is only a small contribution to this process.
Author contributionsPMS proposed the concept of the article. All authors contributed equally to its development and writing and approved the final manuscript for submission.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank the Medical Department of Novartis Farma – Produtos Farmacêuticos S.A, in the person of Dr. Daniel Brás, for their assistance with the bibliographic research.
Please cite this article as: Marques da Silva P, Aguiar C. Sacubitril/valsartan: um importante avanço no puzzle terapêutico da insuficiência cardíaca. Rev Port Cardiol. 2017;36:655–668.