Impaired heart rate (HR) recovery after exercise testing is considered a predictor of cardiovascular mortality as it reflects vagus nerve dysfunction.
ObjectiveTo assess the relationship between body mass index (BMI) and HR recovery after exercise.
MethodsWe analyzed the records of 2443 patients of both sexes, aged between 20 and 59 years, in sinus rhythm, not using negative chronotropic agents and with no myocardial ischemic response to exercise testing carried out at a specialist clinic, between 2005 and 2011. BMI was categorized as normal (18.5–<25 kg/m2), overweight (25–≤30 kg/m2) or obese (>30 kg/m2). The different BMI groups were compared in terms of HR recovery after exercise, which was calculated as the difference between maximum HR during exercise and in the first minute of recovery. Recovery was considered impaired when the difference was ≤12 bpm.
ResultsEighty-seven (3.6%) patients presented impaired recovery, which was three times more prevalent in the obese group and twice as prevalent in the overweight group compared with the normal group (p<0.001 and p=0.010, respectively). Obese patients presented higher basal HR and lower maximum HR, as well as reduced chronotropic reserve (p<0.001). In multivariate analysis, impaired HR recovery was associated with overweight (relative risk [RR]=1.8; p=0.035), obesity (RR=2; p=0.016), number of metabolic equivalents (RR=0.82; p<0.001) and resting HR (RR=1.05; p<0.001). The hazard ratio for hypertension was 2 (p=0.083, NS).
ConclusionImpaired HR recovery was associated with higher BMI, demonstrating that obese individuals present vagus nerve dysfunction.
Declínio atenuado da frequência cardíaca após teste ergométrico é considerado preditor de mortalidade cardiovascular, por refletir disfunção autonômica vagal.
ObjetivoAvaliar a relação entre índice de massa corpórea (IMC) e recuperação da frequência cardíaca após teste ergométrico.
MétodosForam incluídos registros de 2.443 pacientes de ambos os sexos, entre 20-59 anos, em ritmo sinusal, sem uso de cronotrópicos negativos e sem resposta isquêmica miocárdica ao teste ergométrico realizado em clínica especializada, entre 2005-2011. O IMC foi categorizado como: normal (18,5 kg/m22), sobrepeso (25 kg/m22) e obeso (IMC >30 kg/m2). A recuperação da frequência cardíaca após esforço, obtida pela diferença entre a máxima no esforço e no 1.° minuto da recuperação, foi comparada entre grupos de IMC. Foi considerada atenuada quando ≤12 bpm.
ResultadosOitenta e sete (3,6%) pacientes registraram recuperação atenuada, sendo três vezes maior no grupo de obesos e duas vezes no de sobrepeso, quando comparados ao grupo adequado (p<0,001, p=0,010, respectivamente). Obesos apresentaram maior frequência cardíaca basal e menor máxima, além de menor reserva cronotrópica (p<0,001). Na análise multivariada, identificou-se influência dessa atenuação por sobrepeso (RR=1,8; p=0,035), obesidade (RR=2,0; p=0,016), MET (RR=0,82; p<0,001) e frequência cardíaca de repouso (RR=1,05; p<0,001). A razão de risco da hipertensão arterial sistêmica igualou-se a dois, sem significância (p=0,083).
ConclusãoA recuperação atenuada da frequência cardíaca associou-se a maiores IMC, corroborando o fato de que obesos apresentam disfunção autonômica vagal.
Cardiovascular disease is more prevalent in obese individuals.1 One possible pathophysiological mechanism behind this association is altered cardiovascular autonomic regulation, including reduced parasympathetic activity. This is an independent risk factor for coronary artery disease as well as a predisposing factor for arrhythmias and sudden death in obese patients2; it appears to precede the development of cardiovascular disease and is thus considered an early risk marker.3 The mechanisms by which weight gain reduces parasympathetic tone have yet to be fully clarified. One possible explanation is that obesity is associated with chronic inflammation of adipose tissue.4–6 Inflammatory adipokines secreted by white fat, such as tumor necrosis factor alpha and interleukin-6, affect the cardiac autonomic balance via the central nervous system, promoting sympathetic hyperactivity, especially in hypertensive obese individuals,7,8 counteracting increased parasympathetic activity and acetylcholine levels, which inhibit release of these inflammatory cytokines.9 It is through inflammatory states and autonomic nervous system dysfunction that obese patients have a higher risk of morbidity and mortality.10
Cardiac autonomic function can be assessed by heart rate recovery (HRR) following exercise testing, a slow decline indicating reduced parasympathetic reactivation.11 Individuals who present impaired HRR after exercise have an almost four-fold greater risk of mortality.12 Furthermore, there is evidence of an association between cardiovascular risk factors and changes in cardiac autonomic regulation.9,13–15
Given that obesity is an important risk factor for cardiovascular disease and that vagal autonomic dysfunction may be an early cardiovascular risk marker, it has been suggested that obese individuals undergoing treadmill exercise testing using a ramp protocol would present impaired HRR after exercise compared to those with normal body mass index (BMI). This makes exercise testing particularly valuable, since it is one of the first exams to be performed during diagnostic investigation and may lead to early identification of patients at greater cardiovascular risk.
The aim of this study was to assess the relationship between BMI and HRR following exercise.
MethodsThis was a cross-sectional study based on secondary data on exercise tests carried out at a specialist clinic in Recife, Brazil, between 2005 and 2011. We included individuals aged between 20 and 59 years, with BMI >18.5 kg/m2, no cardiovascular disease, and not using negative chronotropic agents, who underwent exercise testing to assess functional capacity or for diagnostic purposes and presented a test duration of ≥7 min.
Exclusion criteria were non-sinus rhythm on ECG, atrioventricular or intraventricular conduction disturbances, and myocardial ischemic response to exercise testing demonstrated by ST-segment abnormalities (upsloping depression of ≥1.5 mm or horizontal or downsloping depression of ≥1 mm).16
The resulting study population consisted of 2443 individuals, who were divided into three groups according to BMI: normal (18.5–<25 kg/m2), overweight (25–≤30 kg/m2) and obese (>30 kg/m2). Age-groups were defined in 10-year intervals.
Heart rate (HR) was recorded after a 10-min rest period, under electrocardiographic monitoring with the Ergo PC 13 system (Micromed®, Brasilia). This was also used during treadmill exercise testing, which was performed using a ramp protocol, initial speed being set at 50% of maximum speed predicted for age and gender,17 with increments of 0.1 km/h every 10 s and an initial incline of 10% less than maximum, increasing by 0.5% every 30 s.
In the active recovery phase, patients walked with the treadmill horizontal at 50% of the speed attained during peak exercise, decreasing by 10% every 30 s until the treadmill came to a complete halt, as described by Silva and Sobral Filho.17
Resting HR with the patient supine and maximum HR at peak exercise were recorded, reserve HR being defined as the difference between the two.
HRR, the study's outcome variable, was defined as the difference between maximum HR and HR during the first minute of the active recovery phase, and was classified as impaired if ≤12 bpm, in accordance with Cole et al.12
Peak oxygen consumption (VO2max), the best measure of exercise capacity, was obtained indirectly using the American College of Sports Medicine's formula,18 expressed in metabolic equivalents (METs).
Statistical analysisThe data were analyzed using Stata 12.1 SE software, the distribution of absolute and relative frequencies being used to describe categorical variables, and measures of distribution and dispersion (means and standard deviation) for numerical variables.
The three BMI groups were compared in terms of age and the exercise test parameters of resting, maximum, reserve and recovery HR and METs using ANOVA and the Tukey test for multiple comparisons. The chi-square test and the Marascuilo procedure were used to analyze gender distribution and the presence of diabetes or hypertension. In addition, Poisson regression analysis with robust variance was used to identify possible predictors of impaired HRR through the calculation of prevalence ratios. The statistical analysis was carried out in two stages, with univariate analysis being used to identify variables with p≤0.20, which were then included in the multivariate analysis. A model was constructed using the backward stepwise method, using p≥0.05 as the exclusion criterion at each step. Each excluded variable was then reintroduced and its statistical significance re-assessed. The final model comprised only variables with p<0.05.
The project was approved by the institution's Ethics Committee and registered as CAAE no. 0194.236.000-11. The study was performed in the Health Sciences Postgraduate Center of Universidade Federal de Pernambuco.
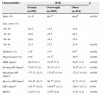
ResultsOf the 2443 patients in the study population, 1380 (56.5%) were male, 484 (19.8%) were hypertensive, 61 (2.5%) were diabetic, and 38 (1.6%) were both diabetic and hypertensive; mean age was 41.2±10.5 years. Mean duration of the exercise test was 9.5±1.4 min (coefficient of variation: 14.7%). The characteristics of the study groups are shown in Table 1.
Characteristics of the study population according to body mass index category.
| Characteristics | BMI | p | ||
|---|---|---|---|---|
| Normal (n=959) | Overweight (n=1050) | Obese (n=434) | ||
| Male (%) | 41.4a | 66.1b | 66.6b | <0.001 |
| Age, years (%) | ||||
| 20–29 | 24.3 | 12.5 | 12.0 | |
| 30–39 | 22.6 | 25.5 | 26.7 | |
| 40–49 | 29.8 | 34.5 | 33.6 | |
| 50–59 | 23.3 | 27.5 | 27.6 | <0.001 |
| Diabetes (%) | 1.0a | 2.4a | 6.0b | <0.001 |
| Hypertension (%) | 9.9a | 21.1b | 38.5c | <0.001 |
| HRR (bpm)† | 26.9a (8.1) | 25.4b (7.7) | 24.0c (7.1) | <0.001 |
| Resting HR (bpm)† | 75.6a (12.2) | 76.3a (11.7) | 78.9b (11.3) | <0.001 |
| Maximum HR (bpm)† | 177.2a (12.3) | 175.0b (13.4) | 172.2c (14.8) | <0.001 |
| Reserve HR (bpm)† | 101.6a (15.7) | 98.7b (15.8) | 93.2c (17.3) | <0.001 |
| HR1 (bpm)† | 150.3b (14.8) | 149.6ab (15.1) | 148.1a (15.1) | 0.043 |
| METs† | 13.6a (3.4) | 12.9b (2.9) | 11.6c (2.3) | <0.001 |
BMI: body mass index; HR: heart rate; HR1: heart rate during first min of recovery; HRR: heart rate recovery; METs: metabolic equivalents.
Although individuals in the overweight and obese groups were older than those with normal BMI, the difference reached statistical significance only in the 50–59 age-group (ANOVA: p<0.001). Men predominated in the overweight and obese groups, and hypertension and diabetes were also more prevalent in these groups (p<0.001). Significant differences between the groups were found in all exercise test parameters (Table 1).
With regard to exercise capacity, Table 2 shows that the groups were homogeneous in terms of percentage of age-predicted maximum HR attained.
Distribution of the study population in terms of maximum heart rate, age-predicted maximum heart rate, and percentage of predicted heart rate attained, according to body mass index category.
| BMI | n | Maximum HR (bpm) | Predicted maximum HR (bpm) | % predicted maximum HR attained |
|---|---|---|---|---|
| Normal | 959 | 175.87±15.04 | 179.47±12.56 | 98.08±6.49 |
| Overweight | 1050 | 184.38±12.10 | 185.68±11.53 | 99.39±4.67 |
| Obese | 434 | 185.60±11.11 | 188.45±10.41 | 98.58±4.88 |
BMI: body mass index; HR: heart rate.
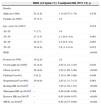
Impaired HRR after exercise was observed in 87 individuals (3.6%; 95% confidence interval 2.8–4.3%), and was three times more prevalent in the obese group (p<0.001) and twice as prevalent in the overweight group (p=0.010) than in those with normal BMI; it was also twice as common in hypertensives (p<0.001) and 2.4 times more prevalent in those with diabetes (p=0.048). Gender was not a risk factor for impaired HRR (Table 3).
Univariate analysis of risk for impaired heart rate recovery in the first minute.
| HRR ≤12 bpmn (%) | Unadjusted RR (95% CI) | p | |
|---|---|---|---|
| Gender | |||
| Male (n=1380) | 52 (3.8) | 1.14 (0.75–1.74) | 0.530 |
| Female (n=1063) | 35 (3.3) | 1.0 | |
| Age, years (n=2443) | 0.014 | ||
| 20–29 | 7 (1.7) | 1.0 | |
| 30–39 | 21 (3.5) | 2.1 (0.9–4.8) | 0.091 |
| 40–49 | 29 (3.7) | 2.2 (0.9–4.9) | 0.063 |
| 50–59 | 30 (4.8) | 2.8 (1.3–6.4) | 0.012 |
| BMI | <0.001 | ||
| Normal (n=959) | 19 (2.0) | 1.0 | |
| Overweight (n=1050) | 42 (4.0) | 2.02 (1.18–3.45) | 0.010 |
| Obese (n=434) | 26 (6.0) | 3.02 (1.69–5.40) | <0.001 |
| Diabetesa (n=61) | 5 (8.2) | 2.38 (1.00–5.66) | 0.048 |
| Hypertensiona (n=484) | 29 (6.0) | 2.02 (1.31–3.13) | 0.001 |
| Resting HR (n=2443)b | – | 1.05 (1.04–1.07) | <0.001 |
| Maximum HR (n=2443)b | – | 0.98 (0.96–0.99) | 0.008 |
| Reserve HR (n=2443)b | – | 0.95 (0.94–0.97) | <0.001 |
| METs (n=2443)b | – | 0.82 (0.75–0.89) | <0.001 |
BMI: body mass index; HR: heart rate; HRR: heart rate recovery; METs: metabolic equivalents; RR: relative risk.
A greater risk of impaired HRR was found in overweight or obese individuals aged 50–59 years who presented a higher resting HR. Protective factors were higher maximum and reserve HR and greater exercise capacity as assessed by number of METs (Table 3) compared to those with normal HRR.
Only variables with p<0.20, and thus potential predictors of impaired HRR, were included in the multivariate analysis: age, BMI, hypertension, resting HR and number of METs. In the final model the significant variables were BMI, resting HR and number of METs. Obesity was the strongest risk marker (RR=1.96), while hypertension was not statistically significant (p>0.05). An increase of one MET was associated with an 11% reduction in risk of impaired HRR, while an increase of one unit in resting HR was associated with a 5% increase (Table 4).
Multivariate analysis of risk for impaired heart rate recovery in the first minute after exercise.
| Unadjusted RR (95% CI) | pa | Adjusted RR (95% CI) | p | |
|---|---|---|---|---|
| Age, years | 0.014 | 0.596 | ||
| 20–29 | 1.0 | 1.0 | ||
| 30–39 | 2.08 (0.9–4.8) | 0.091 | 1.59 (0.68–3.71) | 0.283 |
| 40–49 | 2.17 (0.9–4.9) | 0.063 | 1.59 (0.70–3.62) | 0.269 |
| 50–59 | 2.82 (1.3–6.4) | 0.012 | 1.80 (0.78–4.17) | 0.170 |
| BMI | <0.001 | 0.043 | ||
| Normal | 1.0 | 1.0 | ||
| Overweight | 2.02 (1.18–3.45) | 0.010 | 1.74 (1.02–2.96) | 0.042 |
| Obese | 3.02 (1.69–5.40) | <0.001 | 1.96 (1.13–3.42) | 0.017 |
| Hypertension | 0.001 | 0.158 | ||
| Yes | 2.02 (1.31–3.13) | 0.001 | 1.35 (0.89–2.06) | |
| No | 1.0 | 1.0 | ||
| Resting HR | 1.05 (1.04–1.07) | <0.001 | 1.05 (1.03–1.06) | <0.001 |
| METs | 0.82 (0.75–0.89) | <0.001 | 0.89 (0.82–0.97) | 0.009 |
BMI: body mass index; CI: confidence interval; HR: hear rate; METs: metabolic equivalents; RR: relative risk.
The present study showed that HRR after exercise was inversely associated with BMI, which is thus a risk factor, and showed a direct correlation with exercise capacity, which is a protective factor.
Other cross-sectional and prospective studies have demonstrated a direct or indirect relation between obesity and impaired HRR after exercise, in both the presence19,20 and absence21,22 of cardiovascular risk factors.
A study of 325 healthy adults aged 18-66 years, with mean BMI <23 kg/m2 (21±2.0 kg/m2 in men and 22.6±1.82 kg/m2 in women), assessed the association between indices of obesity (BMI, waist circumference and waist-to-hip ratio) and HRR after exercise and showed significant and independent associations.22 Assoumou et al.,23 using Holter ECG monitoring to assess the correlation between overall and abdominal obesity and HR variability, demonstrated that the two indices were significantly associated with reduced HR variability in both the time and frequency domains. The authors argued that increased abdominal fat, independently of overall obesity, compromises the autonomic nervous system, since reduced HR variability indicates reduced vagal activity. This supports the findings of a study of 125 obese individuals, with no history of stroke or cardiovascular events or use of regular medication undergoing an exercise and weight loss program, which demonstrated that weight loss was associated with improved HRR, indicating increased vagal tone.21 Such evidence corroborates the finding of the present study that there is an association between BMI and impaired HRR, but does not explain it.
One explanation was provided in a study by Vieira et al.,24 who found that lower levels of C-reactive protein were associated with more rapid HRR in older sedentary individuals. They argued that the parasympathetic nervous system is involved in regulating chronic inflammation.
In our study, individuals who presented impaired HRR were generally older, but age was not a predictive factor. By selecting only those aged up to 59, we sought to eliminate age as a confounding factor, since from age 60 onwards there is a more rapid decline in parasympathetic modulation,25 which may be explained by subclinical sinus node dysfunction due to changes in calcium channels, resulting in decreases in sinus node depolarization reserve.26
Diabetes was associated with a higher risk of impaired HRR in our study. Brinkwort et al.,19 in a prospective study assessing overweight and obese men, mean age 46.5±1.3 years, following a weight-loss program based on diet restriction with no modification of physical activity, found that the best predictors of improvement in HRR were reductions in weight and plasma glucose concentrations.
Hypertension was associated with impaired HRR on univariate analysis, but was not significant on multivariate analysis. An explanation for this finding is to be found in the population-based CARDIA study,27 in which cross-sectional analysis showed that impaired HRR was associated with increases in blood pressure; however, in the 15-year follow-up cohort, impaired HRR was not associated with the development of hypertension.
Of the parameters assessed during exercise testing, resting HR showed an inverse correlation with HRR, even after adjustment for age, BMI, hypertension and number of METs. This is explained by the fact that resting HR is under inhibitory parasympathetic control, while HRR immediately after exercise is modulated by parasympathetic reactivation.28 Both are therefore used as markers of cardiac autonomic balance, as demonstrated in various studies.13,15,29
In our study, patients with impaired HRR had lower exercise capacity than those with more rapid recovery. Other studies have demonstrated that increased oxygen consumption is associated with improved HRR.13,15 Exercise, particularly aerobic, affects autonomic nervous system balance by increasing parasympathetic tone and decreasing sympathetic activity29 and improves peak oxygen consumption. However, obesity itself affects HRR, as shown by Gondoni et al.,30 who compared the HR behavior of trained and untrained obese individuals to that of individuals with normal BMI during exercise testing, and concluded that obese subjects, regardless of their fitness level, presented slower HRR.
One finding of our study was that the obese group showed greater exercise capacity than was expected, which may be explained by various factors; firstly, only individuals who achieved an exercise test duration of ≥7 min were included, this being considered the best estimate of peak oxygen consumption31; secondly, the maximum HR attained was close to that predicted for age; and lastly, most of the population consisted of healthy individuals, reducing the likelihood of poor performance.
Among the study's limitations are its retrospective nature, the fact that medications that could affect HR behavior were not considered in the analysis, and patients’ normal levels of physical activity were not specified. However, these limitations do not invalidate the results.
ConclusionThe study showed an association between obesity and impaired HRR after exercise, which may be of value in the early identification of individuals at risk of cardiovascular events. The data presented here, together with those in the literature, support inclusion of HR behavior among the parameters assessed in exercise testing.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Academic linksThis article is based on Tereza Cristina Barbosa Lins's Master's dissertation at Universidade Federal de Pernambuco.
Please cite this article as: Barbosa Lins TC, Valente LM, Sobral Filho DC, Barbosa e Silva O. Relação entre a frequência cardíaca de recuperação após teste ergométrico e índice de massa corpórea. Rev Port Cardiol. 2014. http://dx.doi.org/10.1016/j.repc.2014.07.006