The aim of this study was to document clinical practice in Portugal regarding the use of electronic cardiac devices in patients with heart failure (HF) and reduced left ventricular ejection fraction (LVEF).
MethodsThe Síncrone study was an observational prospective multicenter registry conducted in 16 centers in Portugal between 2006 and 2014. It included adult patients with a diagnosis of HF, LVEF <35% and indication for implantable cardioverter-defibrillator (ICD) and/or cardiac resynchronization therapy (CRT) devices, according to the recommendations of the European Society of Cardiology at the beginning of the study. Patients were followed for one year according to the practice of each center.
ResultsA total of 486 patients were included in the registry, half of whom received an ICD and the other half a CRT pacemaker (CRT-P) or CRT defibrillator (CRT-D). Mean age was 65±12 years and the most frequent causes of HF were ischemic (47%) and idiopathic dilated cardiomyopathy (28%). Overall mortality at one year was 3.6% and the hospitalization rate was 11%, significantly higher in patients with CRT-P/CRT-D than with ICD (17% vs. 5.6%, p<0.001). Patients who received CRT-P/CRT-D experienced significant reductions in QRS duration (160±21 vs. 141±24 ms, p<0.001) as well as improvement in New York Heart Association functional class.
ConclusionThe Síncrone study shows that the use of implantable devices in HF with reduced LVEF in Portugal is in accordance with international recommendations and that patients presented functional improvement and reduced one-year mortality.
O objetivo deste registo foi caracterizar a prática clínica em Portugal no que respeita à utilização de dispositivos eletrónicos na terapêutica dos doentes com insuficiência cardíaca (IC) e fração de ejeção ventricular esquerda (FEVE) diminuída.
MétodosO estudo Síncrone é um registo observacional, multicêntrico e prospetivo que foi conduzido em 16 centros portugueses de 2006 a 2014. Incluiu doentes adultos com diagnóstico de IC, FEVE<35% e indicação para implantação de dispositivos de desfibrilhação (CDI) e/ou ressincronização (CRT), de acordo com as recomendações da Sociedade Europeia de Cardiologia em vigor aquando do início do estudo. Os doentes foram seguidos durante um ano, de acordo com a prática de cada centro.
ResultadosNo total, foram incluídos 486 doentes, dos quais metade implantou CDI e a outra metade CRT-P ou CRT-D. A idade média foi de 65±12 anos e as etiologias mais frequentes foram a isquémica (47%) e a miocardiopatia dilatada idiopática (28%). A mortalidade global ao ano foi 3,6% e a taxa de reinternamento 11%, sendo significativamente maior nos doentes que implantaram CRT-D/CRT-P em relação aos que implantaram CDI (17% versus 5,6% p<0,001). Nos doentes submetidos a CRT observou-se redução significativa da duração do QRS (160±21ms versus 141±24ms, p<0,001), bem como melhoria da classe funcional New York Heart Association.
ConclusãoO estudo Síncrone mostra que a utilização dos dispositivos implantáveis na IC com FEVE diminuída está de acordo com as recomendações internacionais, tendo os doentes apresentado melhoria funcional e reduzida mortalidade ao ano.
Despite advances in pharmacological therapy for patients with heart failure (HF) and reduced left ventricular ejection fraction (LVEF) between the late 1980s and the early 2000s, HF continues to cause high morbidity and mortality, while its prevalence continues to rise.1 Among non-pharmacological treatments investigated in the last 15 years, growing interest has been shown in electronic cardiac devices, namely implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT) devices, the latter including CRT pacemakers (CRT-P) and CRT defibrillators (CRT-D).
Implantation of an ICD in patients with reduced LVEF and signs of HF is a class I recommendation in the European Society of Cardiology (ESC) heart failure guidelines,2 leading to demonstrated improvement in survival (although underused in Portugal).3,4 Multiple studies assessing the impact of CRT on morbidity and mortality in this population have shown significant reductions in risk of death or hospitalization,5,6 as well as improvements in symptoms, functional capacity, quality of life and survival.
The presence of QRS complexes with duration ≥120 ms and a pattern of complete left bundle branch block (LBBB) is the most frequently used criterion to identify patients with ventricular dyssynchrony who could benefit from CRT.2 However, this criterion has its limitations, and a third of patients do not respond to the therapy.7 This failure has been attributed to factors including lead placement, myocardial characteristics such as fibrosis and viability in areas of late contraction, and absence of mechanical dyssynchrony.
Some studies have suggested that other methods, particularly echocardiography, can provide additional markers of ventricular dyssynchrony,8 and at the beginning of the Síncrone registry it was thought that this exam could by itself identify candidates for CRT. Uncertainty regarding this question led to successive revisions of the international guidelines over the years, and the search goes on for criteria with sufficient sensitivity and specificity to select patients who would benefit most from CRT.9 The debate concerning the usefulness of echocardiographic parameters in the selection process continues.
We therefore set out to perform an observational study, collecting data on clinical practice in Portugal and assessing the outcomes of the use of cardiac electronic devices to treat patients with HF and LVEF <35%.
MethodsStudy designThe Síncrone study was an observational prospective multicenter registry conducted in 16 Portuguese centers. It was sponsored by the Portuguese Institute of Cardiac Rhythm (IPRC) and its steering committee included members of the IPRC and of the Working Groups on Echocardiography and Heart Failure of the Portuguese Society of Cardiology.
Centers invited to participate in the registry were those with appropriate capacity, facilities and human resources in the three relevant aspects of cardiology: HF (clinical assessment of patients, optimization of pharmacological therapy and referral), echocardiography (identification of patients with LVEF <35% and assessment of dyssynchrony-related parameters), and arrhythmology (assessment of arrhythmic risk and indication for device implantation) (Supplementary Table 1).
In centers that agreed to participate, the study protocol was submitted to the local ethics committee and the administrative board of the appropriate hospital, and patient recruitment began only after it was accepted by both these bodies.
All patients provided written informed consent for participation in the study and for the use of the data collected for research purposes.
Population and follow-upThe study included patients of both sexes aged 18 years or over with a diagnosis of HF, LVEF <35% and indication for ICD and/or CRT. The data were entered by the investigators at each center in an electronic database housed at the IPRC and accessible via the internet. Five time points for patient assessment were defined: before device implantation, at hospital discharge, and at three, six and 12±1 months after implantation, for a total of one year. Follow-up was conducted according to the practice of each center.
At each assessment, demographic, clinical, laboratory, therapeutic, radiological, echocardiographic, arrhythmic and electrophysiological data were to be provided. The echocardiographic parameters to be assessed were those recommended in the joint guidelines of the American Society of Echocardiography and the European Association of Echocardiography,10 and included criteria of atrioventricular, interventricular and intraventricular dyssynchrony (Supplementary Table 1).
In the initial study design patients were to be recruited for one year from the time of the first inclusion in each center. Difficulties in some centers in recruiting patients led to this period being extended and the steering committee eventually decided that the study would end when a total of 500 patients had been enrolled.
Study endpointsThe primary combined endpoint of the Síncrone study was all-cause mortality and rehospitalization up to one year following device implantation.
Secondary endpoints were mortality and hospitalization due to HF. The study also aimed to determine patients’ clinical, electrophysiological and echocardiographic characteristics, to identify predictors of response to CRT, to analyze in-hospital and outpatient complications and their predictors, and to assess clinical practice in Portugal regarding implantation of ICDs and CRT devices.
Statistical analysisContinuous variables were expressed as mean ± standard deviation and were compared using the Student's t test or the non-parametric Mann-Whitney test. Normality of distribution was assessed using the Kolmogorov-Smirnov test.
Categorical variables were expressed as number and percentage and were compared using the chi-square test and/or Fisher's exact test.
Logistic regression models were used to estimate odds ratios of predictors of clinical outcomes with 95% confidence intervals. The variables with p<0.01 on univariate analysis used to assess dyssynchrony as a predictor of clinical improvement in multivariate analysis were diabetes, intraventricular dyssynchrony and QRS duration.
A p value <0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS version 22.0.
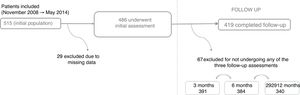
ResultsBetween November 2008 and May 2014, a total of 515 patients were enrolled in the registry. Of these, 486 underwent the baseline assessment, 29 having been excluded due to missing data on the case report form, and 419 completed follow-up, 67 having been lost by this stage of the study (Figure 1).
Baseline characteristicsTable 1 shows the baseline characteristics of the study population. Mean age was 65±12 years and 77% were male. The most frequent causes of HF were ischemic (47%) and idiopathic dilated cardiomyopathy (28%). Patients were under maximally tolerated optimal pharmacological therapy, with 76% taking angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), 77% taking beta-blockers and 34% taking aldosterone antagonists.
Baseline characteristics of the study population.
| Total (n=486) | ICD (n=243) | CRT (n=243) | p | |
|---|---|---|---|---|
| Age, years | 65±12 | 63±13 | 68±11 | <0.001 |
| Male, n (%) | 358 (77) | 198 (84) | 160 (70) | <0.001 |
| BMI, kg/m2 | 27±5 | 27±5 | 27±4 | 0.66 |
| Cardiovascular risk factors, n (%) | ||||
| Hypertension | 291 (60) | 139 (57) | 152 (63) | 0.23 |
| Diabetes | 140 (29) | 58 (24) | 82 (34) | 0.01 |
| Dyslipidemia | 246 (51) | 123 (51) | 123 (51) | 1.0 |
| Etiology of HF, n (%) | ||||
| Ischemic | 229 (47) | 144 (59) | 85 (35) | <0.001 |
| Idiopathic DCM | 135 (28) | 35 (14) | 100 (41) | <0.001 |
| Dilated hypertrophy | 32 (6.6) | 31 (13) | 1 (0.4) | <0.001 |
| Hypertensive | 51 (11) | 27 (11) | 24 (10) | 0.77 |
| Alcoholic | 22 (4.5) | 10 (4.1) | 12 (4.9) | 0.66 |
| Other | 56 (12) | 21 (8.6) | 35 (14) | 0.047 |
| CKD, n (%) | 58 (12) | 20 (8,2) | 38 (16) | 0.01 |
| Anemia, n (%) | 41 (8.4) | 15 (6.2) | 26 (11) | 0.07 |
| Previous AF, n (%) | 111 (23) | 53 (22) | 58 (24) | 0.59 |
| Previous VT/VF, n (%) | 109 (22) | 80 (33) | 29 (12) | <0.001 |
| Hospitalized in previous year, n (%) | 69 (14) | 28 (12) | 41 (17) | 0.09 |
| NYHA class (n=414), n (%) | n=184 | n=230 | ||
| I | 55 (13) | 41 (22) | 14 (6.1) | <0.001 |
| II | 212 (51) | 115 (63) | 97 (42) | <0.001 |
| III | 140 (34) | 27 (15) | 113 (49) | <0.001 |
| IV | 7 (1.7) | 1 (0.5) | 6 (2.6) | 0.11 |
| Medication (n=349), n (%) | n=157 | n=192 | ||
| ACEI/ARB | 266 (76) | 108 (69) | 158 (82) | 0.003 |
| Beta-blocker | 267 (77) | 114 (73) | 153 (80) | 0.12 |
| Aldosterone antagonist | 119 (34) | 36 (23) | 83 (43) | <0.001 |
| Diuretic | 252 (72) | 93 (59) | 159 (83) | <0.001 |
| Statin | 212 (61) | 102 (65) | 110 (57) | 0.14 |
| Anticoagulant | 118 (34) | 49 (31) | 69 (36) | 0.35 |
| Antiplatelet | 198 (57) | 101 (64) | 97 (50) | 0.01 |
| Antiarrhythmic | 72 (21) | 33 (21) | 39 (20) | 0.87 |
ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AF: atrial fibrillation; BMI: body mass index; CKD: chronic kidney disease; CRT: cardiac resynchronization therapy device; DCM: dilated cardiomyopathy; HF: heart failure; ICD: implantable cardioverter-defibrillator; NYHA: New York Heart Association; VT/VF: ventricular tachycardia/ventricular fibrillation.
Around 14% of the population had been hospitalized at least once in the previous year and 22% had a documented episode of ventricular tachycardia/ventricular fibrillation. In these cases the device was implanted for secondary prevention of sudden cardiac death.
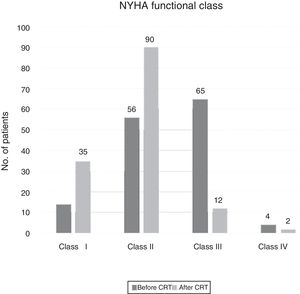
Of the patients who underwent the initial assessment, half (n=243) received an ICD and the other half a CRT-D (n=208) or a CRT-P (n=35). Compared to those with CRT devices, those with ICDs were younger and more often male, and had fewer comorbidities such as diabetes or chronic kidney disease, and most (85%) were in New York Heart Association (NYHA) class I or II, while most (91%) with CRT devices were in NYHA class II or III.
Mean left ventricular ejection fraction (LVEF) was 28.7±8.5%, and right ventricular systolic function was preserved in most cases. Patients with CRT devices had greater ventricular volumes and lower LVEF (Table 2).
Baseline echocardiographic characteristics of the study population.
| n=486 | ICD (n=243) | CRT (n=243) | p | |
|---|---|---|---|---|
| LVEF, % | 28.7±8.5 | 30.6±9.6 | 27.3±7.4 | 0.002 |
| LVEDD, mm | 65.9±9.8 | 62.9±9.7 | 67.9±9.3 | <0.001 |
| LVESD, mm | 53.9±13.0 | 51.2±13.3 | 55.9±12.4 | 0.01 |
| LVEDD index, ml/m2 | 103.9±32.2 | 94.6±20.9 | 113.9±43.4 | <0.001 |
| LVESD index, ml/m2 | 74.1±30.5 | 66.5±19.7 | 78.4±33.9 | 0.08 |
| E/E’ ratio | 15.7±11.0 | 14.8±5.2 | 16.2±13.2 | 0.50 |
| PASP, mmHg | 41.6±12.0 | 40.7±12.2 | 42.1±11.9 | 0.55 |
| TAPSE, mm | 18.3±4.6 | 18.2±4.6 | 18.4±4.7 | 0.58 |
CRT: cardiac resynchronization therapy device; ICD: implantable cardioverter-defibrillator; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; PASP: pulmonary artery systolic pressure; TAPSE: tricuspid annular plane systolic excursion.
In patients with CRT devices, the mean QRS interval was 160±21 ms; in 73% QRS was ≥150 ms, 89% had complete LBBB, and 24% (n=58) had atrial fibrillation (Supplementary Table 2 and Supplementary Figures 1 and 2).
Clinical outcomesOverall one-year mortality, the study's primary outcome, was 3.6% (15 patients), and all-cause rehospitalization was 11% (46 patients) (Table 3). Cardiovascular mortality was 1.9% and the main cause for rehospitalization at one year was HF (4.5%), followed by procedure-related complications (2.6%) and arrhythmias (1.4%). Patients with CRT devices presented higher cardiovascular mortality (3.4% vs. 0.5%, p=0.028) and more were rehospitalized (17% vs. 5.6%, p<0.001) than those with ICDs. There was a trend toward higher overall mortality in patients with ischemic etiology (5.4% vs. 1.9%, p=0.05) (Table 3).
Mortality and rehospitalization by device type and heart failure etiology.
| n=419 | ICD (n=214) | CRT (n=205) | p | Ischemic (n=203) | Non-ischemic (n=216) | p | |
|---|---|---|---|---|---|---|---|
| Overall mortality | 15 (3.6%) | 5 (2.3%) | 10 (4.9%) | 0.162 | 11 (5.4%) | 4 (1.9%) | 0.050 |
| CV mortality | 8 (1.9%) | 1 (0.5%) | 7 (3.4%) | 0.028 | 6 (3.0%) | 2 (0.9%) | 0.129 |
| Overall rehospitalization | 46 (11.0%) | 12 (5.6%) | 34 (17%) | <0.001 | 28 (14%) | 18 (8.3%) | 0.074 |
| Rehospitalization for HF | 19 (4.5%) | 5 (2.3%) | 14 (6.8%) | 0.027 | 13 (6.4%) | 6 (2.8%) | 0.075 |
CRT: cardiac resynchronization therapy device; CV: cardiovascular; HF: heart failure; ICD: implantable cardioverter-defibrillator.
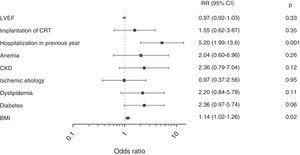
Independent predictors of the primary combined endpoint of all-cause mortality and cardiovascular rehospitalization were at least one hospitalization in the previous year (relative risk [RR] 5.20; 95% confidence interval [CI] 95% CI, 1.99-13.6; p=0.001) and higher body mass index (BMI) (RR 1.14; 95% CI, 1.02-1.26; p=0.02) (Figure 2).
Implantation-related in-hospital complications were uncommon (4.1%; n=17), occurring mainly in patients with CRT devices (n=16). The most frequent were coronary sinus dissection (n=8), significant bleeding (n=5) and lead dislodgement (n=3). At one-year follow-up, device-related complications had been recorded in 8.6%, including problems with lead placement or function (n=17), phrenic nerve stimulation, (n=7), and pocket infection (n=4). Predictors of these complications were the use of CRT devices (RR 3.35; 95% CI, 1.26-8.90; p=0.02) and female gender (RR 3.35; 95% CI, 1.42-7.93; p=0.01).
Of patients with defibrillators (ICD or CRT-D), 71 received shocks (18.1%), most of them appropriate (77%).
Patients with CRT devices experienced significant reductions in QRS duration (160±21 ms baseline vs. 141±24 ms at follow-up, p<0.001) as well as improvement in NYHA functional class in 62% (Figure 3). There were no significant differences in the proportions of patients who presented improvement in NYHA class according to QRS duration (QRS ≥150 ms: 69% vs. QRS 130-149 ms: 67%, p=0.85) (Supplementary Table 3).
In patients with complete baseline echocardiographic assessment (n=82), the only predictor of improvement in NYHA functional class was the presence of intraventricular dyssynchrony (RR 5.23; 95% CI, 1.13-24.3; p=0.035) (Supplementary Figures 1 and 2 and Supplementary Table 3).
DiscussionAlthough patient recruitment for the Síncrone study was extended for several years, during which there were advances in the treatment of HF, we wish to stress that the study's data and conclusions are still highly relevant. The pharmacological treatment prescribed for the study population was generally in line with international standards of practice, as defined in the most recent guidelines,11 and can thus still be considered to constitute optimal therapy (76% taking ACEIs/ARBs, 77% taking beta-blockers and 34% taking aldosterone antagonists). Indications for device placement were also in agreement with current criteria, particularly the option in most cases for a CRT-D.6,12,13 Most (87%) patients receiving CRT devices had complete LBBB and prolonged QRS interval (≥130 ms), which suggests that in Portugal, the class I recommendations for these devices are being followed.11 Nevertheless, in a small proportion of cases implantation was based on class II recommendations. This does not appear to us to be due to funding constraints, but rather to problems with the organization of HF care, calculation of patient flows, and the referral system.14 Better coordination between different areas of HF treatment is therefore required, including the establishment of referral protocols and of multidisciplinary teams to link the different levels of HF care in the country.
The quality of pharmacological treatment regimens, indications, implantation procedures, and follow-up in HF patients in these Portuguese centers may be responsible for the low mortality (only 3.6% at one year) and rehospitalization rate (11%) observed during the study period. One-year mortality (6.4%) and HF hospitalization rate (9.9%) were higher in the European Society of Cardiology Heart Failure Long-Term (ESC-HF-LT) Registry in a population of outpatients with chronic HF, mean LVEF of 37% and in NYHA class I or II.15,16 The lower mortality in our study may be partly related to the loss of patients to follow-up, some of whom may have died. Additionally, the populations of the two registries are completely different: our study enrolled only patients with cardiac devices and severe left ventricular dysfunction, while in the ESC-HF-LT Registry, which included all types of chronic HF, the proportion with ICDs and CRT devices was less than would be expected considering that many patients had reduced LVEF. As the rate of ICD and CRT implantation in the unselected HF population of the ESC-HF-LT Registry was much lower than in our study, it is logical that it would present significantly higher mortality and rehospitalization rates.
Not surprisingly, there was a tendency in our registry for greater mortality and more rehospitalizations in patients with ischemic rather than non-ischemic etiology. It is also logical that hospitalization during the previous year identified more severe patients and was an independent predictor of mortality and cardiovascular rehospitalization. The other independent predictor of one-year mortality, BMI, rekindles the debate on the paradoxical effect of obesity on overall and cardiovascular mortality in HF.17–19
The greater complexity of the implantation procedure for CRT devices explains the higher rate of complications and reintervention in patients with these devices, as seen in other studies.20 Female gender was also a predictor of device-related complications, related to factors including differences in anatomy and smaller body surface area.21,22
The results of CRT in our study can be considered good from a functional standpoint, with improvements in NYHA class seen in 62% of patients, whether of ischemic or non-ischemic etiology. This is comparable to outcomes reported in other studies comparing CRT with medical therapy, which showed improved NYHA class in 60-70% of patients.6,23
Unlike published randomized trials,24,25 the Síncrone study suggests that echocardiographic assessment of intraventricular dyssynchrony may be able to predict response to CRT. It should be noted that our results were obtained in a population in which most patients had LBBB with greatly prolonged QRS interval, which contrasts with the EchoCRT trial.24 Furthermore, unlike the PROSPECT trial,25 in which response to CRT was assessed by a clinical score and reduction in left ventricular end-systolic volume, the only predictor of response used in the Síncrone study was improvement in NYHA functional class. This finding may be due to awareness on the part of echocardiographers following publication of the PROSPECT trial results of the need for greater precision in measurements and more careful selection of the parameters used to assess dyssynchrony.
This potential use of echocardiography is supported by other recent studies that have shown better performance of echocardiographic parameters in predicting clinical improvement in these patients.26–28
Study limitationsThe Síncrone study has several limitations, the chief of which is that it was merely observational and did not require alterations to the participating centers’ clinical practice, which may not have been uniform, and this may have affected the final results.
The asymmetrical distribution of subjects included by the different centers, although reflecting the situation in Portugal, in which a few centers dominate, may have biased the results. The recruitment period was extended beyond that originally stipulated and 67 patients were lost to follow-up, which constitutes a limitation. However, there can be no doubt that the results obtained reflect high-quality clinical practice that incorporated current advances in HF treatment.
It should be borne in mind that participation in the registry was voluntary and that participating centers received no remuneration. The centers’ commitment to the study was repeatedly demonstrated and the professionalism of their approach was unrelated to their size.
When analyzed as a whole, the study's findings show the importance of establishing a systematic approach to the treatment of patients with HF and left ventricular dysfunction, as has been reported in other multicenter studies.
The small number of patients who underwent complete baseline echocardiographic studies, together with the simplicity and subjectivity of the measured outcome, are also limitations of the study.
ConclusionsThe findings of the observational Síncrone study allow us to conclude that in Portuguese centers treating patients with HF and reduced LVEF using implantable cardiac devices, both pharmacological therapy and indications for device implantation are in line with the strictest international standards as outlined in the European guidelines. This is particularly clear from the option to implant CRT-D devices in most cases.
The outcomes of CRT in the study are also comparable to those in the international literature, showing significant improvements in functional class for both ischemic and non-ischemic HF etiology. The quality of care provided by these centers is demonstrated by the fact that their overall mortality was lower than in international registries.
Finally, an interesting and perhaps controversial finding was that the presence of intraventricular dyssynchrony on the echocardiogram predicted response to CRT, as this technique has fallen into disfavor as a method for predicting CRT response since the publication of the PROSPECT trial.25
FundingThe Síncrone study received an unconditional research grant from Boston Scientific Portugal – Dispositivos Médicos, Lda., who were not involved in developing the study protocol, collecting and analyzing the data, or preparing the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank the lead investigators and other workers at the participating centers (listed in Supplementary Table 4); Boston Scientific Portugal for an unconditional research grant to carry out the study; KeyPoint, Consultoria Científica Lda., for their assistance with developing the study protocol, data processing, and monitoring; and Infortucano for their help in creating the centralized electronic database that the centers were able to access via the internet.
Please cite this article as: Bonhorst D, Guerreiro S, Fonseca C, Cardim N, Macedo F, Adragão P. Implantação de dispositivos de ressincronização e/ou desfibrilhação em doentes com insuficiência cardíaca: dados da vida real o Estudo Síncrone. Rev Port Cardiol. 2019;38:33–41.