Pulmonary hypertension (PH) covers a group of conditions characterized by an increase in pulmonary vascular resistance leading to right ventricular failure. Risk stratification is crucial for adequate prognostic and therapeutic assessment. However, the accuracy of conventional parameters is limited, especially biomarkers.
ObjectivesTo determine the prognostic value of new biomarkers and their combination in a multi-biomarker approach to predict outcome in patients with PH.
MethodsIn this prospective cohort study, PH patients underwent clinical, echocardiographic and laboratory assessment, including quantification of serum N-terminal pro-brain natriuretic peptide (NT-proBNP) and of the following new biomarkers: mid-regional pro-adrenomedullin (MR-proADM), copeptin, endothelin-1, mid-regional pro-atrial natriuretic peptide (MR-proANP) and soluble ST2 (sST2), the interleukin-33 receptor. The accuracy of the different parameters for predicting all-cause mortality and death or hospitalization of cardiac causes was determined. The prognostic value of a multi-biomarker score based on the tertile distribution of serum NT-proBNP, MR-proANP, renin and sST2 was compared to conventional markers.
ResultsForty-three patients (72.1% female, age 59±15 years) were included, most of whom (65.1%) had group 1 PH. During a median follow-up of 34 months, 26% of the patients died and 35% were hospitalized for cardiac causes. A trial and ventricular dimensions and right ventricular fractional area change were prognostic predictors. Log NT-proBNP (HR: 31.14; 95% CI: 3.12-310.7; p=0.003) and renin (HR: 1.02; 95% CI: 1.005-1.038; p=0.009) were independent predictors of mortality. MR-proANP (HR: 1.008; 95% CI 1.004-1.011; p<0.001) and sST2 (HR: 1.005; 95% CI 1.001-1.009; p=0.04) were predictors of death or hospitalization. The prognostic value of the multi-biomarker score was higher than any of the conventional parameters, and enabled identification of risk groups (the high-risk group had three-year mortality of 77.8%).
ConclusionA multi-biomarker approach was superior for risk stratification to any single marker. A score that incorporates NT-proBNP, MR-proANP, renin and sST2 accurately identifies patients at low, intermediate and high risk.
A hipertensão pulmonar (HP) compreende um grupo de doenças progressivas caracterizadas por aumento na resistência vascular pulmonar, conduzindo a falência ventricular direita e morte prematura. A estratificação de risco é fundamental para a avaliação prognóstica e orientação terapêutica, sendo que a acuidade dos parâmetros convencionais é limitada, sobretudo no que respeita aos biomarcadores.
ObjetivosDeterminar o valor prognóstico de um painel de novos biomarcadores e avaliar o benefício da sua conjugação num score multibiomarcador para predição de morbimortalidade na HP.
MetodologiaEstudo de coorte prospetivo de doentes com HP submetidos a avaliação clínica, ecocardiográfica e laboratorial, incluindo doseamento da porção N-terminal do péptido natriurético cerebral (NT-proBNP) e dos seguintes novos biomarcadores: porção médio-regional da pro-adrenomedulina (MR-proADM), copeptina, endotelina-1, porção médio-regional da pró-hormona ANP (MR-proANP) e recetor solúvel da interleucina-33 (sST2). Determinou-se a acuidade dos diferentes parâmetros de avaliação na predição de morte por qualquer causa e de morte ou hospitalização por causa cardíaca. Concebeu-se um score multibiomarcador a partir da distribuição por tercis dos níveis séricos de novos biomarcadores e a sua acuidade prognóstica incremental foi avaliada comparativamente aos marcadores convencionais.
ResultadosIncluíram-se 43 doentes com HP (72,1% sexo feminino; 59±15 anos). A maioria dos doentes (65,1%) tinha HP, grupo 1. Durante um seguimento mediano de 34 meses, 26% dos doentes (n=11) faleceram e 35% (n=15) foram hospitalizados por causa cardíaca. Os diferentes parâmetros dimensionais ventriculares e auriculares, bem como a menor fração de área ventricular direita, foram preditores prognósticos relevantes. Relativamente aos biomarcadores, foram preditores independentes de mortalidade o NT-proBNP (log) (hazard ratio [HR]: 31,14; intervalo de confiança a 95% [IC95%]: 3,12-310,7; p=0,003) e a renina (HR: 1,02; IC95%: 1,005-1,038; p=0,009), e do risco de morte ou hospitalização o MR-proANP (HR: 1,008; IC95% 1,004-1,011; p<0,001) e o sST2 (HR: 1,005; IC95% 1,001-1,009; p=0,04). O score multibiomarcador, concebido a partir da distribuição por tercis dos níveis séricos de NT-proBNP, MR-proANP, renina e sST2, foi superior a qualquer dos parâmetros convencionais na estratificação prognóstica e possibilitou a identificação dos grupos de baixo risco, risco intermédio e risco elevado, cuja mortalidade aos três anos atingiu 77,8%.
ConclusãoUma abordagem multibiomarcadores é útil na estratificação prognóstica de doentes com HP. O score que incorpora o NT-proBNP, MR-proANP, renina e sST2 identifica com precisão os doentes de elevado risco.
Pulmonary hypertension (PH) covers a group of conditions characterized by an increase in pulmonary vascular resistance leading to right ventricular (RV) failure and premature death. Various pathological processes are involved in progression to heart failure (HF) secondary to PH, particularly myocardial injury, vascular remodeling and neurohormonal activation due to right chamber pressure overload. Risk stratification is crucial for prognostic and therapeutic assessment, as well as for the prescription and titration of new vasodilators. Stratification is usually based on exercise capacity, echocardiography, and hemodynamic assessment by cardiac catheterization.1 However, the value of many of these parameters is limited, as they are subjective in nature, operator-dependent and/or invasive.
In recent years the prognostic value of several biomarkers has been investigated in PH2 that may provide an efficient and noninvasive means for risk stratification and monitoring. In the international guidelines, only measurement of brain natriuretic peptide or its N-terminal portion (NT-proBNP) is recommended in everyday practice.1 Nevertheless, the value of NT-proBNP is limited for prognostic stratification, particularly in the presence of concomitant left heart disease or renal failure. Furthermore, various studies have suggested that significant elevation of serum NT-proBNP only occurs late, at a stage when there is marked ventricular dilatation and adjustment of therapy may no longer be effective.3
Given the complexity of the mechanisms underlying PH, the value of a multi-biomarker approach is being investigated. Clinical research has mainly focused on prognostic stratification of patients with left HF, and the most promising biomarkers have only been tested in small groups of PH patients with right HF, which limits the validity of the results. Several biomarkers studied in left HF reflect biological processes that may be of more relevance in progression of PH, particularly those related to pulmonary vascular remodeling (endothelial dysfunction, thrombosis in situ and oxidative stress) and RV overload. There is thus a pressing need to investigate biomarkers such as soluble ST2 (sST2, the interleukin-33 receptor), plasma renin, adrenomedullin and endothelin-1 (ET-1).
sST2 is a biomarker with multiple effects in vivo. Its expression is upregulated in cardiac fibroblasts and cardiomyocytes exposed to mechanical strain.4 Studies have documented increased serum sST2 levels in acute HF and suggest a correlation with remodeling and fibrosis in chronic HF.5 However, its prognostic value in right HF secondary to PH is unknown.
Various studies have demonstrated that chronic upregulation of the renin-angiotensin-aldosterone system (RAAS) in patients with left HF has significant prognostic implications, but its relevance in right HF secondary to PH is uncertain and the predictive value of plasma renin has never been established in this patient group.
Adrenomedullin, a vasoactive peptide with natriuretic properties produced in various tissues, has been consistently associated with prognosis in left HF,6 but its role in prognostic stratification of PH patients has yet to be determined.
ET-1 is a potent vasoconstrictor and a therapeutic target in PH.7 Serum ET-1 levels are increased in various stages of HF, but studies with longer follow-up are needed to assess its prognostic impact.
The aim of the present study was to determine the prognostic value of new biomarkers and to assess the benefit of combining them in a multi-biomarker approach to predict outcome in right HF secondary to PH.
MethodsStudy designThis was a prospective observational study of consecutive patients referred to a multidisciplinary PH clinic with a diagnosis of PH, confirmed by hemodynamic assessment. Patients with group I, III, IV or V PH on the 2013 Nice classification8 who had been clinically stable for the previous two months and were in World Health Organization (WHO) functional class ≥II were included. The exclusion criteria were PH secondary to left heart disease, PH consistent with chronic respiratory disease, history of surgery or trauma in the previous two months, malignancy, cirrhosis of the liver or a history of myocardial infarction. The time between diagnosis of the disease or institution of specific therapy and referral to the PH clinic (and hence inclusion in the study) was not taken into account. The study design was approved by the hospital's ethics committee and all participants gave their informed consent.
Baseline assessment of the patients included clinical and echocardiographic evaluation and laboratory tests, including measurement of new and conventional biomarkers.
Laboratory testsSerum and plasma samples were taken in EDTA blood tubes from the antecubital vein, after a five-minute rest period. A complete blood count and serum concentrations of creatinine (from which glomerular filtration rate [GFR] was calculated by the Modification of Diet in Renal Disease formula9), sodium and urea were determined. Plasma aliquots were stored at -80°C for subsequent analysis of biomarkers. Levels of NT-proBNP were measured using the IMMULITE 2000 solid phase immunometric assay (Siemens Healthcare Diagnostics, Breda, the Netherlands), which has been validated in clinical practice. C-terminal ET-1,10 mid-regional pro-atrial natriuretic peptide (MR-proANP),11 mid-regional pro-adrenomedullin (MR-proADM),12 copeptin13 and sST214 were quantified by chemiluminescence assay using the Kryptor® system (BRAHMS AG, Hennigsdorf, Germany).
Transthoracic echocardiographyEchographic exams were performed with a GE Vivid 7 Dimension® ultrasound system, and included conventional two-dimensional, M-mode, Doppler and pulsed tissue Doppler study of the lateral tricuspid annulus. The digital images were post-processed and analyzed using EchoPAC Dimension® software (GE Healthcare, Milwaukee, WI), with observers blinded to the patient's identity. RV systolic function was assessed by fractional area, tricuspid annular plane systolic excursion (TAPSE) and peak lateral tricuspid annular systolic velocity (TASV).
Clinical follow-upPatients attended in-person consultations every 2-3 months, during which changes in symptoms, need to adjust therapy and adverse clinical events, including hospitalization for cardiac causes or death, were recorded.
The study's endpoints were defined as: (1) all-cause mortality; and (2) death or hospitalization due to worsening PH during follow-up.
Statistical analysisCategorical variables were expressed as absolute and relative frequencies and compared using the chi-square test and Fisher's exact test. Continuous variables with normal distribution were expressed as means and standard deviation and compared using the Student's t test and ANOVA, while those with non-normal distribution were expressed as medians and interquartile range (IQR) and compared by the Mann-Whitney and Kruskal-Wallis nonparametric tests. Given that NT-proBNP values were not normally distributed, they were logarithmically transformed before inclusion in the survival analysis.
Kaplan-Meier survival curves and univariate and multivariate Cox regression analysis were used to determine the association of different variables with mortality and death or hospitalization of cardiac causes during long-term follow-up. Event-free survival was compared on the basis of the log-rank p value, hazard ratio (HR) and respective 95% confidence interval (CI). In order to avoid undue weight being attributed to inconclusive associations, only variables with p<0.10 on univariate analysis were included in the multivariate analysis. In addition, multivariate analyses were performed using the forward stepwise conditional method, with inclusion probability of 5% and exclusion probability of 10%.
The accuracy of the different parameters for prognostic stratification was assessed by the area under the receiver operating characteristic curve (AUC), CIs and p values being calculated for mortality and death or hospitalization of cardiac causes at three years. A multi-biomarker score was calculated for each patient based on the tertile distribution of serum NT-proBNP, MR-proANP, renin and sST2 levels. The prognostic accuracy of this score was then compared to that obtained with the various biomarkers and echocardiographic parameters considered separately, and the impact on prognosis was assessed based on the tertile distribution of the score.
ResultsPopulation characteristicsForty-three patients were studied, of whom 72.1% were female (n=31), median age 59 years (IQR: 43-67). All presented symptoms of HF on initial assessment, 74.% (n=32) and 25.6% (n=11) being classified as in WHO functional class II and III, respectively. With regard to etiology, the majority of patients (65.1%; n=28) had group 1 PH (2013 Nice classification), most frequently associated with connective tissue disease (n=11) or congenital heart disease (n=9). In addition, 83.7% (n=36) were under specific vasodilator therapy, mainly phosphodiesterase type 5 inhibitors (58.1%; n=25) and endothelin receptor antagonists (55.8%; n=24). The clinical characteristics of the study population are detailed in Table 1.
Population characteristics.
| n (%) | |
|---|---|
| Clinical Classification | |
| Group 1 | 28 (65.1%) |
| Idiopathic | 7 (16.3%) |
| Associated with connective tissue disease | 11 (25.6%) |
| Associated with HIV infection | 1 (2.3%) |
| Associated with congenital heart disease | 9 (20.9%) |
| Group 3 | 6 (14.0%) |
| Secondary to interstitial lung disease | 4 (9.3%) |
| Secondary to mixed obstructive and restrictive pulmonary disease | 2 (4.7%) |
| Group 4 (chronic thromboembolic PH) | 7 (16.3%) |
| Group 5 (PH with unclear multifactorial mechanisms) | 2 (4.7%) |
| Specific vasodilator therapy at initial assessment | 36 (83.7%) |
| Phosphodiesterase inhibitors | 25 (58.1%) |
| Endothelin receptor antagonists | 24 (55.8%) |
| Calcium channel blockers | 5 (11.6%) |
HIV: human immunodeficiency virus; PH: pulmonary hypertension.
Patients underwent echocardiographic assessment, details of which are shown in Table 2, a median of eight days (IQR: 2-91) after laboratory tests. The main findings were that 51.2% (n=22) presented RV dilatation (baseline diameter >42 mm) and that 86% (n=37) had at least one parameter of RV systolic dysfunction: reduced fractional area (<35%) in 41.9% (n=18), reduced TAPSE (<16 mm) in 34.9% (n=15) and reduced peak lateral TASV (≤10 cm/s) in 39.5% (n=17). In addition, right atrial (RA) dilatation (maximum diameter >53 mm) was observed in 51.2% (n=22).
Echocardiographic parameters at initial assessment.
| Right atrium | |
| Diameter (4-chamber), mm | 56±14 |
| Diastolic area, cm2 | 22.5 (14.1-33.8) |
| Systolic area, cm2 | 16.5 (9.3-25.8) |
| Fractional area, % | 21.4 (11.1-35.8) |
| Right ventricle | |
| Basal diameter, mm | 42.9 (37.7-54.0) |
| Diastolic area, cm2 | 22.5 (18.8-35.0) |
| Systolic area, cm2 | 15.5 (10.0-20.1) |
| Fractional area, % | 37.4±12.1 |
| TAPSE, mm | 18.7 (15.8-23.0) |
| Lateral TASV, cm/s | 11.0 (9.0-12.5) |
| Estimated PASP, mmHg | 64.6 (46.0-84.7) |
PASP: pulmonary artery systolic pressure; TAPSE: tricuspid annular plane systolic excursion; TASV: tricuspid annular systolic velocity.
Laboratory parameters are shown in Table 3. Although renal failure was common, only mild (GFR 60-89 ml/min in 24%, n=24) or moderate (GFR 30-59 ml/min in 25.6%, n=11) dysfunction was identified. Patients with RV dilatation presented higher serum NT-proBNP (1338 [354-2275] vs. 236 pg/ml [111-1005], p=0.021) and MR-proANP (159.7 [132.6-287.9] vs. 111.1 pg/ml [66.1-161.9], p=0.032), while those with reduced RV fractional area had higher NT-proBNP (1338 [822.0-2393.5] vs. 236 pg/ml [186-1258], p=0.019); and those with RA dilatation had elevated serum NT-proBNP (1522 [825-2462] vs. 215 pg/ml [91-719], p<0.001), MR-proANP (163.5 [152.6-287.9] vs. 94.9 V [50.8-127.4]. p<0.001) and sST2 (62.2 [38.3-77.0] vs. 30.1 pg/ml [24.2-47.9], p=0.015).
Laboratory parameters at initial assessment.
| Urea (mg/dl) | 45±16 |
| Creatinine (mg/dl) | 0.87 (0.75-1.07) |
| GFR (MDRD) (ml/min) | 72 (59-82) |
| Cystatin C (mg/l) | 0.82 (0.76-1.09) |
| Sodium (mmol/l) | 140±3 |
| ACE (μg/ml) | 719 (190-1695) |
| Renin (mIU/l) | 15.5 (4.5-50.8) |
| Aldosterone (pg/ml) | 91.7 (47.4-181.6) |
| NT-proBNP (pg/ml) | 719 (190-1695) |
| MR-proANP (pmol/l) | 151.9 (93.5-183.9) |
| Copeptin (ng/ml) | 9.1 (5.0-15.0) |
| ET-1 (pg/ml) | 82.7 (62.9-122.1) |
| MR-proADM (pg/ml) | 0.86±0.5 |
| sST2 (pg/ml) | 47.7 (27.1-75.5) |
ACE: angiotensin-converting enzyme; ET-1: endothelin-1; GFR: glomerular filtration rate; MDRD: Modification of Diet in Renal Disease formula; MR-proADM: mid-regional pro-adrenomedullin; MR-proANP: mid-regional pro-atrial natriuretic peptide; sST2: soluble ST2.
During a median follow-up of 34 months (30-38), 26% of the patients died (n=11) and 35% (n=15) were hospitalized for cardiac causes. Pharmacological therapy was adjusted, phosphodiesterase inhibitors being introduced in nine patients (20.9%), endothelin receptor antagonists in 14 (32.6%) and parenteral prostanoids in five (11.6%), so that all patients were being medicated with at least one vasodilator.
The 11 patients who died during follow-up had greater RV and RA dilatation and reduced RV fractional area on initial echocardiographic assessment (Table 4). The various ventricular and atrial dimensions, as well as reduced RV fractional area, were important predictors of mortality. Of the echocardiographic parameters, the independent predictors of risk of death identified by multivariate logistic regression analysis were RV basal diameter (HR: 1.17, 95% CI: 1.07-1.27, p<0.001) and RA fractional area (HR: 0.87, 95% CI: 0.79-0.97, p=0.008).
Association of clinical, laboratory and echocardiographic variables with mortality during follow-up.
| Clinical course | Multivariate Cox regression analysis | |||||
|---|---|---|---|---|---|---|
| Death (n=11) | Favorable course (n=32) | p | Hazard ratio | 95% CI | p | |
| Demographic characteristics | ||||||
| Age, years | 63 (43-72) | 56 (40-66) | NS (0.42) | 1.01 | 0.98-1.05 | NS (0.74) |
| Female, n (%) | 7 (63.6) | 24 (75.0) | NS (0.47) | 0.57 | 0.17-1.97 | NS (0.57) |
| Clinical characteristics | ||||||
| Group 1 PH | 9 (81.8) | 19 (59.4) | NS (0.071) | 0.23 | 0.03-1.98 | NS (0.18) |
| Groups 3-5 PH | 2 (18.2) | 13 (40.6) | NS (0.071) | 4.36 | 0.51-37.72 | NS (0.18) |
| Echocardiographic characteristics | ||||||
| RA diameter (4-chamber), mm | 71±11 | 51±11 | <0.001 | 1.08 | 1.03-1.13 | 0.001 |
| RA diastolic area, cm2 | 38.8 (28.7-50.2) | 19.0 (13.0-24.3) | <0.001 | 1.04 | 1.01-1.06 | 0.007 |
| RA systolic area, cm2 | 31.0 (25.7-48.0) | 13.2 (8.1-20.1) | <0.001 | 1.09 | 1.04-1.14 | <0.001 |
| RA fractional area, % | 10.5 (6.9-10.9) | 25.0 (14.0-39.8) | 0.001 | 0.88 | 0.80-0.97 | 0.013 |
| RV basal diameter, mm | 57.8 (54.1-64.9) | 39.3 (36.0-45.1) | <0.001 | 1.12 | 1.06-1.20 | <0.001 |
| RV diastolic area, cm2 | 40.0 (25.1-49.0) | 21.2 (16.8-28.7) | 0.004 | 1.06 | 1.01-1.11 | 0.012 |
| RV systolic area, cm2 | 26.7 (16.8-33.4) | 12.2 (9.6-18.3) | 0.003 | 1.08 | 1.02-1.13 | 0.005 |
| RV fractional area, % | 30±11 | 40±12 | 0.033 | 0.94 | 0.88-0.99 | 0.033 |
| RV: TAPSE, mm | 16 (13-23) | 20 (16-23) | NS (0.19) | 0.92 | 0.82-1.05 | NS (0.22) |
| RV: Lateral TASV, cm/s | 10 (6-11) | 12 (9-13) | 0.026 | 0.80 | 0.61-1.06 | NS (0.12) |
| Estimated PASP, mmHg | 88 (67-96) | 61 (46-76) | 0.013 | 1.02 | 0.99-1.04 | NS (0.07) |
| Laboratory characteristics | ||||||
| GFR (MDRD) (ml/min) | 58 (44-72) | 76 (66-84) | 0.002 | 0.94 | 0.90-0.99 | 0.01 |
| Cystatin C (mg/l) | 1.01 (0.77-1.30) | 0.80 (0.75-1.08) | NS (0.13) | 6.84 | 0.63-73.95 | NS (0.11) |
| Sodium (mmol/l) | 139±4 | 140±3 | NS (0.25) | 0.86 | 0.71-1.05 | NS (0.15) |
| ACE (μg/ml) | 30 (24-51) | 31 (21-42) | NS (0.59) | 1.01 | 0.99-1.04 | NS (0.39) |
| Renin (mIU/l) | 55.2 (16.2-142.5) | 10.8 (3.0-23.1) | 0.004 | 1.01 | 1.004-1.02 | 0.001 |
| Aldosterone (pg/ml) | 138.0 (31.2-391.0) | 87.7 (48.1-143.8) | NS (0.29) | 1.00 | 0.999-1.001 | NS (0.49) |
| Log NT-proBNPa (pg/ml) | 2854 (1326-5632) | 316 (174-1163) | <0.001 | 9.44 | 2.40-37.13 | 0.001 |
| MR-proANP (pmol/l) | 1.24±0.45 | 0.73±0.45 | 0.006 | 1.004 | 1.002-1.007 | 0.001 |
| Copeptin (ng/ml) | 18.84 (13.09-25.16) | 6.14 (5.00-10.29) | <0.001 | 1.1 | 1.04-1.16 | 0.001 |
| ET-1 (pg/ml) | 142.8±62.9 | 76.5±38.2 | 0.014 | 1.01 | 1.005-1.02 | 0.004 |
| MR-proADM (pg/ml) | 1.19 (0.93-1.40) | 0.62 (0.47-0.85) | 0.001 | 3.06 | 1.19-7.87 | 0.021 |
| sST2 (pg/ml) | 75.5 (56.7-103.5) | 40.1 (25.4-59.1) | 0.002 | 1.002 | 0.997-1.007 | NS (0.48) |
Serum values were logarithmically transformed for the logistic regression analysis.
ACE: angiotensin-converting enzyme; ET-1: endothelin-1; GFR: glomerular filtration rate; MDRD: Modification of Diet in Renal Disease formula; MR-proADM: mid-regional pro-adrenomedullin; MR-proANP: mid-regional pro-atrial natriuretic peptide; NS: non-significant; NT-proBNP: N-terminal pro-brain natriuretic peptide; PASP: pulmonary artery systolic pressure; PH: pulmonary hypertension; RA: right atrium; RV: right ventricle; sST2: soluble ST2; TAPSE: tricuspid annular plane systolic velocity; TASV: tricuspid annular systolic velocity.
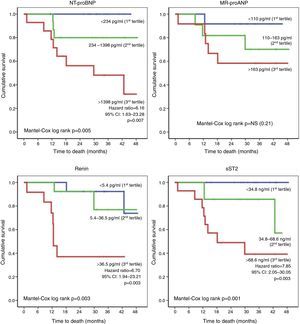
With regard to laboratory parameters, higher levels of NT-proBNP, MR-proANP, renin, copeptin, ET-1, MR-proADM and sST2 were observed in patients who died during follow-up (Table 4). In addition, the risk of death was greater in those with worse renal function. Of the various biomarkers studied, only log NT-proBNP and renin were independent predictors of mortality (HR: 31.14 [3.12-310.7], p=0.003 and HR: 1.02 [1.005-1.038], p=0.009, respectively). The risk of death during follow-up was determined on the basis of the tertile distribution of serum levels of the biomarkers (Figure 1). Compared to patients in the first and second tertiles, those with higher levels of NT-proBNP (third tertile: >1398 pg/ml) had a greater risk of death (HR: 6.16, 95% CI: 1.63-23.28, p=0.007), as did those with elevated renin (third tertile: >36.5 pg/ml; HR: 6.70, 95% CI: 1.94-23.21, p=0.003) and sST2 (third tertile: >68.6 ng/ml; HR: 7.85, 95% CI: 2.05-30.05, p=0.003).
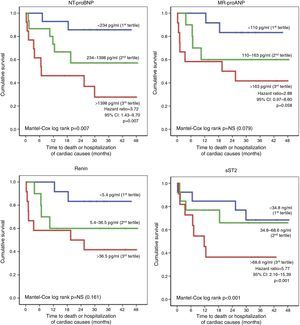
Patients who had an unfavorable clinical course (death or hospitalization of cardiac causes, n=18) also presented more changes in right-chamber dimensions and function on initial echocardiographic assessment (Table 5), but the only independent echocardiographic predictor of this endpoint was RV basal diameter (HR: 1.08, 95% CI: 1.04-1.12, p<0.001). Furthermore, these patients also showed elevated serum NT-proBNP, MR-proANP, renin, copeptin, ET-1, MR-proADM and sST2 levels, which were identified as significant prognostic markers. Initial GFR values did not significantly affect the risk of death or hospitalization. Of the biomarkers studied, MR-proANP (HR: 1.008, 95% CI: 1.004-1.011, p<0.001) and sST2 (HR: 1.005, 95% CI: 1.001-1.009, p=0.04) were independent predictors of death or hospitalization. Event-free survival was determined on the basis of the tertile distribution of serum levels of the biomarkers (Figure 2). Compared to the first and second tertiles, the risk of adverse events was three times greater in patients with higher NT-proBNP (third tertile: >1398 pg/ml; HR: 3.72; 95% CI: 1.43-9.70, p=0.007) and six times greater in those with elevated sST2 (third tertile: >68.6 ng/ml; HR: 5.77, 95% CI: 2.16-15.39, p<0.001).
Association of clinical, laboratory and echocardiographic variables with death or hospitalization of cardiac causes during follow-up.
| Clinical course | Univariate Cox regression analysis | |||||
|---|---|---|---|---|---|---|
| Death or hospitalization (n=18) | Favorable course (n=25) | p | HR | 95% CI | p | |
| Demographic characteristics | ||||||
| Age, years | 59 (43-66) | 62 (42-67) | NS (0.95) | 1.003 | 0.97-1.03 | NS (0.87) |
| Female, n (%) | 11 (61.1) | 20 (80.0) | NS (0.17) | 0.49 | 0.18-1.28 | NS (0.14) |
| Clinical characteristics | ||||||
| Group 1 PH | 16 (88.9) | 12 (48.0) | NS (0.26) | 0.16 | 0.02-1.44 | NS (0.11) |
| Groups 3-5 PH | 2 (11.1) | 13 (52.0) | NS (0.26) | 6.23 | 0.70-55.81 | NS (0.11) |
| Echocardiographic characteristics | ||||||
| RA diameter (4-chamber), mm | 65±14 | 50±11 | <0.001 | 1.07 | 1.03-1.11 | <0.001 |
| RA diastolic area, cm2 | 29.8 (23.7-44.9) | 17.5 (12.5-22.9) | 0.001 | 1.02 | 1.003-1.04 | 0.026 |
| RA systolic area, cm2 | 25.0 (19.1-37.2) | 12.3 (8.1-18.7) | 0.001 | 1.06 | 1.02-1.09 | 0.001 |
| RA fractional area, % | 13.7 (9.9-25.5) | 25.5 (13.5-40.6) | 0.018 | 0.95 | 0.90-0.99 | 0.032 |
| RV basal diameter, mm | 54 (48-63) | 38 (32-43) | <0.001 | 1.08 | 1.04-1.12 | <0.001 |
| RV diastolic area, cm2 | 29.4 (23.4-47.5) | 20.0 (16.2-22.5) | <0.001 | 1.06 | 1.02-1.09 | 0.002 |
| RV systolic area, cm2 | 19.2 (16.0-31.7) | 11.0 (8.9-17.2) | <0.001 | 1.08 | 1.03-1.12 | 0.001 |
| RV fractional area, % | 32±9 | 42±13 | 0.01 | 0.94 | 0.90-0.99 | 0.012 |
| RV: TAPSE, mm | 18 (14-23) | 20 (16-23) | NS (0.53) | 0.96 | 0.88-1.06 | NS (0.45) |
| RV: lateral TASV, cm/s | 10 (7-12) | 12 (9-13) | NS (0.17) | 0.92 | 0.76-1.11 | NS (0.36) |
| Estimated PASP, mmHg | 85 (67-97) | 56 (42-72) | <0.001 | 1.02 | 1.01-1.04 | 0.004 |
| Laboratory characteristics | ||||||
| GFR (MDRD) (ml/min) | 66 (52-80) | 75 (65-82) | NS (0.11) | 0.98 | 0.95-1.01 | NS (0.23) |
| Cystatin C (mg/l) | 0.79 (0.74-1.08) | 0.90 (0.76-1.09) | NS (0.68) | 1.72 | 0.17-17.65 | NS (0.65) |
| Sodium (mmol/l) | 140±3 | 140±3 | NS (0.53) | 0.97 | 0.82-1.15 | NS (0.71) |
| ACE (μg/ml) | 29.5 (20.0-49.5) | 32.0 (24.0-42.0) | NS (0.83) | 1.02 | 0.99-1.04 | NS (0.19) |
| Renin (mIU/l) | 38.7 (8.8-84.6) | 9.4 (4.2-23.1) | NS (0.07) | 1.004 | 1.001-1.007 | 0.014 |
| Aldosterone (pg/ml) | 89.8 (39.3-208.7) | 99.5 (48.7-177.8) | NS (0.99) | 1.00 | 0.999-1.001 | NS (0.99) |
| NT-proBNP (log)a (pg/ml) | 1527 (1054-3529) | 236 (161-825) | 0.001 | 5.3 | 1.95-14.39 | 0.001 |
| MR-proANP (pmol/l) | 163.2 (153.3-467.4) | 112.2 (74.3-160.3) | NS (0.056) | 1.01 | 1.004-1.01 | <0.001 |
| Copeptin (ng/ml) | 11.8 (5.0-19.0) | 6.3 (5.0-10.4) | 0.07 | 1.05 | 1.01-1.10 | 0.013 |
| ET-1 (pg/ml) | 118.0±62.3 | 77.2±40.6 | 0.025 | 1.02 | 1.01-1.03 | 0.001 |
| MR-proADM (pg/ml) | 1.02±0.48 | 0.75±0.49 | NS (0.13) | 2.53 | 1.07-5.95 | 0.034 |
| sST2 (pg/ml) | 74.7 (48.2-100.5) | 40.0 (26.6-47.9) | 0.003 | 1.01 | 1.001-1.008 | 0.011 |
Serum values were logarithmically transformed for the logistic regression analysis.
ACE: angiotensin-converting enzyme; ET-1: endothelin-1; GFR: glomerular filtration rate; MDRD: Modification of Diet in Renal Disease formula; MR-proADM: mid-regional pro-adrenomedullin; MR-proANP: mid-regional pro-atrial natriuretic peptide; NS: non-significant; NT-proBNP: N-terminal pro-brain natriuretic peptide; PASP: pulmonary artery systolic pressure; PH: pulmonary hypertension; RA: right atrium; RV: right ventricle; sST2: soluble ST2; TAPSE: tricuspid annular plane systolic velocity; TASV: tricuspid annular systolic velocity.
The stratification analysis suggested not only that the various biomarkers have different prognostic impact but that the time to events and the type of event (mortality vs. death or hospitalization) they predict are also different. Thus, while NT-proBNP and renin showed an association mainly with mortality, MR-proANP and sST2 were more clearly associated with death or hospitalization. We therefore considered the possibility that combining these biomarkers might increase the accuracy of prognostic stratification.
In order to test this hypothesis, we developed a stratification score based on the tertile distribution of serum NT-proBNP, MR-proANP, renin and sST2 levels, the points for each biomarker being adjusted based on the HRs for the prediction of mortality and death or hospitalization of cardiac causes (Table 6). The final score, ranging from 0 to 10, corresponds to the sum of the points for each biomarker.
Multi-biomarker prognostic stratification score.
| Variable | Points |
|---|---|
| NT-proBNP | |
| <234 pg/ml | 0 |
| 234-1398 pg/ml | 2 |
| >1398 pg/ml | 4 |
| MR-proANP | |
| ≤163 pg/ml | 0 |
| >163 pg/ml | 1 |
| Renin | |
| ≤36.5 pg/ml | 0 |
| >36.5 pg/ml | 1 |
| sST2 | |
| <34.8 ng/ml | 0 |
| 34.8-68.6 ng/ml | 2 |
| >68.6 ng/ml | 4 |
| Total score | 0-10 |
MR-proANP: mid-regional pro-atrial natriuretic peptide; NT-proBNP: N-terminal pro-brain natriuretic peptide; sST2: soluble ST2.
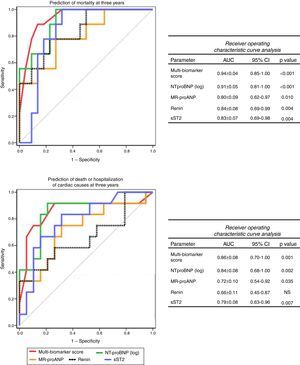
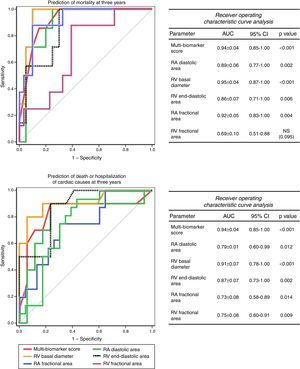
The accuracy of this score for predicting events at three years was high, both for mortality (AUC: 0.94±0.04, 95% CI: 0.85-1.00, p<0.001) and for death or hospitalization of cardiac causes (AUC: 0.86±0.08, 95% CI: 0.70-1.00, p=0.001). Furthermore, its accuracy was superior to any of the biomarkers included considered separately (Figure 3) and to any of the echocardiographic parameters (Figure 4).
Receiver operating curve analysis of biomarkers for prediction of mortality and death or hospitalization of cardiac causes at three years. AUC: area under the curve; CI: confidence interval; MR-proANP: mid-regional pro-atrial natriuretic peptide; NS: non-significant; NT-proBNP: N-terminal pro-brain natriuretic peptide; sST2: soluble ST2.
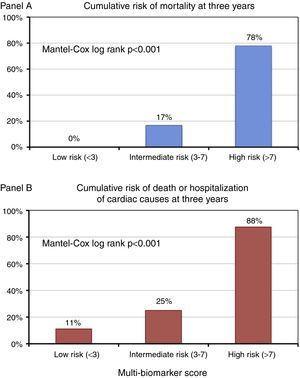
Based on the tertile distribution of the multi-biomarker score, three groups of patients were identified with markedly different three-year prognoses (Mantel-Cox log rank p<0.001): score<3 (low risk), no fatal events at three years and a cumulative rate of death or hospitalization of 11.1%; score 3-7 (intermediate risk), three-year mortality of 16.7% and a cumulative rate of death or hospitalization of 25%; and score >7 (high risk), three-year mortality of 77.8% and a cumulative rate of death or hospitalization of 87.5% (Figure 5).
DiscussionIn this prospective study, we assessed the prognostic accuracy of new biomarkers (MR-proANP, renin, copeptin, ET-1, MR-proADM and sST2) in patients with right HF secondary to PH and compared it to that of conventional echocardiographic parameters. We confirmed the prognostic value of NT-proBNP and demonstrated for the first time that assessment of serum NT-proBNP, MR-proANP, renin and sST2 levels combined in a multi-biomarker score has additional value. The score showed a high degree of accuracy for prognostic stratification, identifying high-risk patients, who may thus benefit from early intensive multidisciplinary therapeutic interventions in the future.
The results of the study indicate that the new biomarkers are valuable prognostic markers and that their clinical application would bring benefits. It is also important to remember that these results were obtained in a population of patients with right HF secondary to PH (confirmed by right heart catheterization) that was relatively heterogeneous in terms of etiology, but the prognostic accuracy of the biomarkers studied did not differ significantly according to the Nice classification. This is of relevance for everyday clinical practice, since prognostic stratification using these biomarkers can be applied to all the various clinical forms of PH.
The study also confirmed that there is a relationship between RA and RV anatomical and functional parameters and prognosis. RV basal diameter and RA fractional area were strong independent predictors of prognosis, whereas RV functional parameters, while also correlating with prognosis, had less impact and were not independent predictors. This highlights the difficulty of echocardiographic assessment of global RV function, which is strongly affected by the altered ventricular-arterial coupling found in PH and possibly dependent on remodeling such as in wall hypertrophy.
Natriuretic peptides, considered the gold standard biomarkers in HF, play an important role in cardiovascular homeostasis, including regulation of vascular tone, blood volume and endothelial permeability, as well as in cardiac hypertrophy. Of these peptides, ANP and BNP, which are mainly produced in the atria and ventricles, respectively, are of particular importance.15 Both are secreted in response to myocardial stretching secondary to pressure or volume overload. The biological functions of ANP and BNP include various compensatory mechanisms, such as natriuresis, diuresis and vasodilation.16
Most studies on B-type peptides have focused on BNP or NT-proBNP. The prognostic value of these peptides has been well documented in all stages of left HR, and is additional to other clinical markers. For example, the ADHERE registry17 found a linear relationship between BNP levels and mortality in patients hospitalized for decompensated HF, even after adjustment for other laboratory or clinical risk markers.
Besides its natriuretic effects, ANP has potent pulmonary vasodilatory properties.18 It has a very short plasma half-life (2-5 min),19 which makes it difficult to measure. Research has thus focused on assessment of MR-proANP, a more biologically stable peptide, whose prognostic value has been assessed in patients with left HF. In the PRIDE study,20 MR-proANP was identified as an independent predictor of mortality in patients with decompensated HF, and the BACH trial21 demonstrated the non-inferiority of MR-proANP compared to BNP in diagnosing acute HF in patients with acute dyspnea; furthermore, data from this trial suggested that MR-proANP had greater accuracy in gray areas of BNP and in obese patients.
The prognostic value of natriuretic peptides has been investigated in the context of PH and RV failure, but the results are limited. Some studies have demonstrated that plasma BNP and NT-proBNP levels increase in proportion to the degree of RV dysfunction22 and reduction in functional capacity,23 but the prognostic value of MR-proANP has not been assessed. The results of our study are therefore of particular interest, suggesting there are advantages to combined assessment of these peptides for risk stratification. NT-proBNP was associated predominantly with mortality (and short-term risk), while MR-proANP was an independent predictor of death or hospitalization (longer-term risk of events). Patients with the highest levels of NT-proBNP (third tertile: >1398 pg/ml) had a six-fold greater risk of death and those in the third tertile for MR-proANP had a three-fold greater risk of death or hospitalization for worsening PH. We also showed that combining these biomarkers increased the accuracy of prognostic stratification. This supports the hypothesis that natriuretic peptides are an integral part of the physiological counter-regulatory response in patients with PH and RV dysfunction, and hence have predictive value.
These results could be of even more interest as these peptides may soon become a therapeutic target. For example, it has been demonstrated that administration of ANP significantly decreased vasoconstriction and adverse remodeling in an animal model of PH.24
Natriuretic peptides have inhibitory effects on the RAAS.25 The prognostic importance of increased RAAS activity is widely accepted in left HF,25 but its impact in right HF is still uncertain. Studies suggest that the RAAS is upregulated in PH as a compensatory mechanism, although this may have long-term negative effects.26 However, little is known about alterations in RAAS signaling in PH. Two separate studies have reported that certain angiotensin-converting enzyme (ACE) and angiotensin II type 1 receptor polymorphisms are associated with more rapid progression of idiopathic PH, suggesting involvement of the RAAS.27,28 Elevated aldosterone levels were recently described as triggering pulmonary vascular disease in experimental models of PH,29 and Maron et al.30 reported that plasma aldosterone levels correlated positively with hemodynamic parameters (pulmonary vascular resistance and transpulmonary gradient) in patients with PH.
Forfia et al.31 demonstrated an association between hyponatremia (an indirect indicator of RAAS activity), right HF and poor survival in patients with PH, but our results did not reveal a significant association between natremia and risk of events after adjustment for other variables. This probably reflects the complexity of the underlying mechanisms and changes in the predictive value of sodium levels due to anticongestive therapy. Our study assessed parameters that are a more direct measure of RAAS activity (ACE, renin and aldosterone), which have not been investigated systematically in long-term follow-up studies. We found that patients who had an unfavorable clinical course presented elevated baseline renin, with no changes in ACE or aldosterone. These results highlight the complexity of the mechanisms involved, not only in terms of modulation of the RAAS itself but also the lack of an association between the extent of RAAS upregulation and disease severity. Possible explanations include the presence of other states of hyperaldosteronism (for example systemic hypertension), inter-individual differences in sodium homeostasis, and circadian factors that regulate aldosterone synthesis.32 Our results point to a potential role for therapies that act on the RAAS.
ET-1 and vasopressin are peptides with vasoconstrictive properties that play a fundamental role in cardiovascular physiology. Besides being a potent vasoconstrictor, ET-1 also has proliferative effects on vascular smooth muscle, and is involved in the pathophysiology of PH. Plasma ET-1 levels are significantly elevated in PH33 and correlate with disease severity,34 but their prognostic value has been little explored. Vasopressin, a peptide hormone secreted by the hypothalamus and stored in the neurohypophysis, has antidiuretic and vasoconstrictive properties. Its levels are elevated in HF,35 but it is difficult to measure due to its short half-life and the fact that it binds to plasma proteins. However, its C-terminal portion (copeptin) is stable and easily measured, and has been studied in the context of HF and acute coronary syndromes. In the BACH study,36 elevated copeptin was associated with higher 90-day mortality and hospitalization for cardiac causes. The prognostic value of copeptin has been demonstrated in both acute and chronic HF.37,38 Compared to left HF, the neurohormonal axis has been little studied in patients with PH and right HF. In our population, we found higher copeptin and ET-1 levels in individuals who had an unfavorable clinical course (death and/or hospitalization of cardiac causes).
ST2 is a member of the Toll-like/IL-1 receptor superfamily, with a soluble (sST2) and a transmembrane (ST2L) isoform; the ligand for both forms is interleukin-33 (IL-33). sST2 is a biomarker with pluripotent effects in vivo, whose expression is upregulated in cardiac fibroblasts and cardiomyocytes exposed to mechanical strain, and plays a role in remodeling and fibrosis in HF. Recent studies have demonstrated higher serum sST2 concentrations in acute HF, correlating significantly with adverse ventricular remodeling and prognosis.4,39 Sanada et al.40 showed that IL-33 acting through ST2L antagonizes myocyte hypertrophy induced by neurohormonal mechanisms. These authors also demonstrated that sST2 inhibits the cardioprotective effect of IL-33. Shimpo et al.41 reported that elevated serum sST2 levels were predictors of HF and mortality in patients with acute myocardial infarction. sST2 has been thoroughly studied in patients with dyspnea and suspected decompensated HF. In a subanalysis of the PRIDE study, Januzzi et al.42 assessed sST2 and NT-proBNP levels in patients with acute dyspnea and demonstrated that sST2 concentrations were higher in those with acute HF and that it had greater prognostic value than NT-proBNP. sST2 has also been shown to be a predictor of sudden cardiac death in patients with chronic HF.43
To summarize, sST2 is now considered a biomarker of severity and prognosis in HF, but few studies have assessed its role in right HF secondary to PH. Zheng et al.44 recently demonstrated a correlation between plasma sST2 levels and hemodynamic parameters in patients with idiopathic PH, while Carlomagno et al.45 reported a correlation between serum sST2 and RV dimensions and function assessed by cardiac magnetic resonance imaging. From a hemodynamic standpoint, patients with RV dysfunction present increased filling pressures, greater myocardial wall stress with ventricular stretching and high sST2 levels. This study consistently demonstrated the value of sST2 as a new prognostic marker in right HF, and given the importance of RV function in risk stratification of patients with PH,46 sST2 can be combined with other biomarkers, as in the risk stratification model proposed in this work.
The adaptation of the right heart to pressure overload and morphological changes arising from PH have been characterized by various imaging modalities, particularly echocardiography. Several studies have identified RA and RV morphological and functional prognostic predictors in these patients.47 Nevertheless, the relationship between anatomical deformation or chamber dilatation and parameters of RV dysfunction has yet to be fully clarified. Furthermore, parameters related to RV dilatation, rather than those related to RV function, appear to be more consistent for determining prognosis in these patients, particularly chamber dimensions and volumes, as assessed by echocardiography and magnetic resonance imaging, respectively.48 Studies of specific therapy in PH patients have found a correlation between these parameters and functional improvement.
LimitationsThe present study should be seen as an exploratory assessment of a relatively small population of patients with right HF secondary to PH confirmed by right heart catheterization, with limited follow-up. Moreover, the study population was heterogeneous in terms of PH etiology, disease duration and therapy at the time of recruitment and during follow-up. Its findings will have to be validated in larger prospective studies before they can be used in clinical decision-making. In addition, the impact of disease duration and therapy on the performance of the different prognostic predictors, both biomarkers and RA and RV anatomical and functional parameters, needs to be assessed.
ConclusionThis prospective study demonstrated that several new biomarkers (MR-proANP, renin, copeptin, ET-1, MR-proADM and sST2), together with NT-proBNP levels and parameters of right chamber dimensions and function, are valuable prognostic predictors in patients with right HF secondary to PH. It also showed for the first time that assessment of serum NT-proBNP, MR-proANP, renin and sST2 levels combined in a multi-biomarker score improves the accuracy of prognostic stratification. The score identified high-risk patients, who may thus benefit from early intensive multidisciplinary therapeutic interventions in the future.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Plácido R, Cortez-Dias N, Robalo Martins S, Almeida AG, Calisto C, Gonçalves S, et al. Estratificação prognóstica na hipertensão pulmonar: valor acrescido da abordagem multibiomarcadores. Rev Port Cardiol. 2017;36:111–125.