To characterize primary malignant cardiac tumors operated on in our center and to analyze patient survival.
MethodsBetween January 1994 and August 2014, 123 patients with cardiac tumors underwent surgery, of which 12 (9.8%) were primary malignant tumors – eight sarcomas (67%), three B-cell lymphomas (25%) and one epithelioid hemangioendothelioma (8.3%). The tumor affected the left atrium in five cases (42%), the right atrium in four (33%), the right ventricle in two (17%) and the pulmonary valve in one (8%). Patients’ mean age was 55.4±16.9 years, 67% were female and 75% presented in NYHA class III–IV.
ResultsResection was complete (negative margins) in five cases and partial in seven (five sarcomas and two lymphomas), and 11 patients needed adjuvant therapy, surgery alone being curative in only one (epithelioid hemangioendothelioma). Mean follow-up was 41.7±61.3 months: 24.8±30.0 months (3.8–95.7) for sarcomas, 70.1±118.0 months (1–206.3) for lymphomas and 91.9 months for the epithelioid hemangioendothelioma. During follow-up, 10 patients died (83%) and two were alive (17%). Overall survival at 30 days, six months, one year and two years was 91.7%, 66.7%, 58.3% and 41.7%, respectively. In the sarcoma group, 1-year and 2-year survival were 62.5% and 37.5%, respectively.
ConclusionsResection of primary malignant cardiac tumors, even partial, is safe, provides relief of obstructive symptoms and improves quality of life, but is rarely curative and has a low survival rate. Due to the rarity of such tumors, a multicenter database could improve knowledge and help clarify the indications for cardiac surgery as a treatment option.
Caracterizar os tumores malignos cardíacos operados no nosso centro e analisar a sobrevida dos doentes.
MétodosDe janeiro/1994 a agosto/2014, 123 doentes com tumores cardíacos foram submetidos a cirurgia, dos quais 12 revelaram ser malignos (9,8%) – oito sarcomas (67%), três linfomas de células B (25%) e um hemangioendotelioma epitelioide (8,3%). A AE estava afetada em cinco casos (42%), a AD em quatro (33%), o VD em dois (17%) e a válvula pulmonar em um (8%). A idade média era 55,4±16,9 anos, 67% do sexo feminino e 75% apresentavam-se em classe III-IV da NYHA.
ResultadosA ressecção foi completa (margens negativas) em cinco casos e parcial em sete (cinco sarcomas, dois linfomas). Onze doentes necessitaram de terapia adjuvante, sendo a cirurgia curativa em apenas um (hemangioendotelioma epitelioide). O tempo de seguimento médio foi de 41,7±61,3 meses; 24,8±30,0 (3,8-95,7 meses) para sarcomas, 70,1±118,0 (1-206,3 meses) para linfomas e 91,9 meses para o hemangioendotelioma epitelioide. Durante o seguimento, dez doentes faleceram (83%). A sobrevida global aos 30 dias, seis meses, um e dois anos foi de 91,7, 66,7, 58,3 e 41,7%, respetivamente. No grupo dos sarcomas, a sobrevida a um e dois anos foi de 62,5 e 37,5%.
ConclusõesA ressecção de tumores malignos primários, mesmo que parcial, é segura, providencia alívio sintomático, podendo melhorar a qualidade de vida, mas é raramente curativa e tem baixa sobrevida. Dada a raridade, uma base de dados multicêntrica poderia melhorar o conhecimento e ajudar a clarificar as indicações cirúrgicas.
Primary cardiac tumors are rare, the incidence on autopsy varying between 0.0017% and 0.03%. Around 25% are malignant and most (75%) are sarcomas.1
Cardiac tumors are generally asymptomatic until they are large enough to cause valve or chamber obstruction. Although they are easily detected by echocardiography, malignancy and specific histological type cannot always be determined or even suspected before surgical intervention.2
The main treatment for primary malignant cardiac tumors, particularly sarcomas, is still complete surgical resection, combined with chemotherapy.3,4 Nevertheless, cardiac sarcomas have a poor prognosis, with mean survival of 11–17 months.1,5,6
The aim of this study was to characterize primary malignant cardiac tumors operated on in our center and to analyze patient survival.
MethodsThis was a retrospective study of patients operated between January 1994 and August 2014. Information was collected from patients’ medical records, together with data on follow-up from hospital records and telephone contact with patients or relatives. During the study period, 23010 patients underwent cardiac surgery. Of these, 123 (0.53%) had cardiac tumors, but only 12 had primary malignant tumors, corresponding to 9.8% of all cardiac tumors and an incidence of 0.05% among patients undergoing cardiac surgery.
Patients’ mean age was 55.4±16.9 years (21–79), 67% were female and 75% presented in NYHA class III–IV. The tumor was located in the left atrium in five cases (42%), the right atrium in four (33%), the right ventricle in two (17%) and the pulmonary valve in one (8%) (Table 1).
Description of the population with primary malignant cardiac tumors.
| M/F | Age | Type of tumor | Location | Resection/surgical marginsa | Associated procedures | Secondary location on preoperative assessment | Morbidity | Adjuvant therapy | Recurrence | Current state | Follow-up (months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | B-cell lymphoma | RA | Partial | No | Pericardial effusion | No | Post-op CT | Heart | Deceased | 3 |
| 2 | F | 64 | B-cell lymphoma | RV | Complete/unspecified | Tricuspid valvuloplasty | No | No | Post-op CT | No | Alive | 206 |
| 3 | M | 58 | B-cell lymphoma | RA | Partial | No | Vena cava syndrome | No | Post-op CT | Heart | Deceased | 1 |
| 4 | F | 64 | Myxofibrosarcoma | LA | Complete/negative | No | Pericardial effusion | No | Post-op CT | Heart | Deceased | 96 |
| 5 | M | 36 | Rhabdomyosarcoma | LA | Complete/negative | LA bovine patch | No | No | Post-op CT + RT | Bone and brain metastases | Deceased | 18 |
| 6 | F | 59 | Undifferentiated sarcoma | LA | Partial | No | No | No | Post-op CT | Heart | Deceased | 6 |
| 7 | F | 51 | Angiosarcoma | RA | Complete/negative | No | Pericardial effusion + ganglion metastases | No | Neoadjuvant CT + post-op CT | Heart and lymph nodes | Deceased | 13 |
| 8 | M | 68 | Leiomyosarcoma | RV | Complete/positive | RA bovine patch | Lung metastases | AF | Post-op CT | Lung | Deceased | 27 |
| 9 | F | 34 | Angiosarcoma | RA | Partial | No | Liver metastases | No | Post-op CT | Heart | Deceased | 27 |
| 10 | F | 68 | Myxochondrosarcoma | LA | Partial | Mitral valvulo plasty | Pericardial effusion | No | Post-op CT | Heart | Deceased | 4 |
| 11 | F | 79 | Angiosarcoma | Pulmonary valve | Complete/positive | Pulmonary homograft | No | AF | Post-op CT | No | Deceased | 7 |
| 12 | F | 21 | Epithelioid hemangioendothelioma | LA | Complete/negative | LA bovine patch | No | No | No | No | Alive | 92 |
Resection: macroscopic surgical resection; Surgical margins: negative means no tumor cells on histological analysis of the margins of the surgical specimen, while positive indicates the presence of tumor cells.
AF: atrial fibrillation; F: female; LA: left atrium; M: male; Post-op CT: post-operative chemotherapy; RA: right atrium; RT: radiotherapy; RV: right ventricle.
Eight of the tumors (67%) were sarcomas, three (25%) B-cell lymphomas and one (8.3%) epithelioid hemangioendothelioma. In the sarcoma group, there were three cases of angiosarcoma (38%) and one each of rhabdomyosarcoma, leiomyosarcoma, myxofibrosarcoma, undifferentiated sarcoma and myxochondrosarcoma.
Only two patients had a histological diagnosis prior to surgery: one with an epithelioid hemangioendothelioma, previously biopsied by right thoracotomy, who had no known metastases and had not undergone neoadjuvant therapy; and the other with an angiosarcoma, diagnosed by mediastinoscopy, with lymph node metastasization and pericardial effusion, who had undergone neoadjuvant chemotherapy, resulting in reduction of the mass and resolution of the pericardial effusion. All the other tumors were diagnosed through anatomopathological analysis of the surgical specimens.
The myxochondrosarcoma, myxofibrosarcoma and one of the lymphomas presented with pericardial effusion. One patient with angiosarcoma had liver metastases preoperatively and the patient with leiomyosarcoma presented diffuse lung metastases. One of the lymphomas presented as vena cava syndrome. The other cases had no known secondary locations. Surgical intervention was prompted by cardiac valve or chamber obstruction in all cases except that of the epithelioid hemangioendothelioma, which was causing no functional impairment.
All patients were operated under cardiopulmonary bypass, in cardiac arrest following administration of cardioplegia and cooling to 28°C. The venae cavae were ligated in all cases that required the right atrium to be opened.
Statistical analysisContinuous variables were expressed as means ± standard deviation and compared using the Student's t test. Survival (time from surgery to death or last recorded follow-up) was analyzed using the Kaplan-Meier method. A value of p<0.05 was considered statistically significant. The data were analyzed using IBM SPSS Statistics®, version 22.
ResultsResection was complete (entire macroscopic tumor excised) in seven cases, although subsequent anatomopathological analysis showed positive margins in two of these (one angiosarcoma and the leiomyosarcoma). In the other five patients (three sarcomas and two lymphomas), it was macroscopically partial because complete resection was technically impossible and/or the main aim was to relieve cardiac valve or chamber obstruction and to obtain material for histological diagnosis.
One patient required a pulmonary homograft, one mitral valvuloplasty (partial posterior annuloplasty), three atrial reconstruction with a bovine pericardial patch (one the left atrial roof, one the left atrial posterior wall and one the right atrial free wall) and one tricuspid valvuloplasty (reimplantation of the anterior leaflet and annuloplasty). There was no need for associated procedures in the other six patients. The procedures performed are shown by patient in Table 1.
All patients survived the operation and none required inotropic support. During hospital stay, the patient with epithelioid hemangioendothelioma had to be reoperated on the fifth postoperative day to repair a small perforation in the mitral valve posterior leaflet. Two other patients had episodes of atrial fibrillation, resolved with medical therapy. There were no major complications such as myocardial infarction, stroke or acute renal failure.
All patients were discharged. Mean follow-up was 41.7±61.3 months overall, 24.8±30.0 months (3.8–95.7) for sarcomas and 70.1±118.0 months (1–206.3) for lymphomas. Ten patients (83%) died during follow-up and two (17%) were alive at the end of follow-up: one with epithelioid hemangioendothelioma, who had no signs of recurrence after 92 months; and another with B-cell lymphoma, who had been in remission for 206 months.
Eleven patients underwent adjuvant therapy (neoadjuvant chemotherapy in one, adjuvant chemotherapy in all eleven, and radiotherapy in one who presented late bone metastases). The surgery was curative in two patients: as the only treatment in the case of epithelioid hemangioendothelioma (complete resection, with negative surgical margins); and associated with adjuvant chemotherapy in one of the patients with B-cell lymphoma (complete resection, with unspecified surgical margins).
The other two patients with lymphoma, in whom the surgical aim was to biopsy the mass and relieve cardiac chamber obstruction, died following aggressive recurrence (one within 30 days of surgery and the other after three months), despite the fact that both had begun chemotherapy.
All eight patients with sarcoma underwent adjuvant chemotherapy, of whom six had local recurrence, irrespective of negative margins or secondary location on pre-operative assessment, and who subsequently died. The patient with rhabdomyosarcoma suffered bone and brain metastasization but no local recurrence, and died of metastatic disease 18 months later. Another, with angiosarcoma but no signs of recurrence, died of pneumonia after seven months.
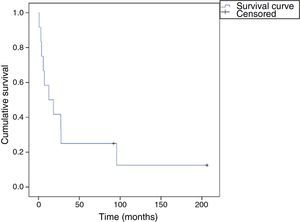
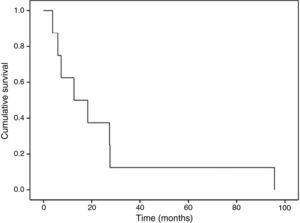
Overall survival at 30 days, six months, one year and two years was 91.7%, 66.7%, 58.3% and 41.7%, respectively (Figure 1). In the sarcoma group, survival was 62.5% at one year and 37.5% at two years (Figure 2), and there was a significant difference (p=0.01) in mean survival between those with partial resection and/or positive margins (14.4±12.0 months) and those with negative surgical margins (42.2±46.4 months). Finally, patients with angiosarcoma tended to present worse mean survival that those with other types of sarcoma (15.8±10.5 vs. 30.2±37.8 months), but the difference was not statistically significant (p=0.25).
DiscussionA review of the literature shows that there are few institutional series of a significant number of patients with primary malignant cardiac tumors,1,5,7,8 most of which are exclusively sarcomas.1,5 There are two multicenter studies on sarcomas – one American, of 27 cases of cardiac sarcoma9 and one by the French Sarcoma Group, with 124 patients.6 Other publications are mainly case reports10 or very small series.3
Only 9.8% of the cardiac tumors treated in our center were malignant, lower than the 25% incidence reported in the literature.3,6 This may be explained by the fact that a significant number of patients had tumors that were too advanced for surgery. With regard to sarcomas, the incidence in our study was 67%, which is close to the 75% generally observed.
The most common sarcoma was angiosarcoma, with most located in the right atrium, as reported in the literature. All three of our patients were women, but given the small number of cases, no gender predominance can be assumed, and other studies have observed no gender difference.1,5,6 Patients with angiosarcoma had a mean survival of 15.9±10.5 months, similar to the 14 months reported by Randhawa et al.,5 but only slightly better than the average of death within 9–12 months without surgical resection.11
Comparison of survival in the sarcoma group according to surgical margins showed that those with histologically negative margins had better outcomes, the difference being statistically significant, which is in agreement with other studies.1,6 This highlights the importance of extemporaneous histological examination and intraoperative assessment of surgical margins in future cases, as well as use of more aggressive surgical techniques such as autotransplantation. If a tumor is not resectable by conservative surgery, orthotopic transplantation may be considered,6,9 although this option is controversial. However, even partial resection is beneficial in the case of sarcomas, as it can provide immediate improvement in the patient's condition and help to control tumor growth.6
According to the literature, primary cardiac lymphomas are rare and most respond well to radiotherapy and chemotherapy, and thus surgical resection is only rarely indicated. However, surgery can provide material for analysis when other diagnostic techniques have been inconclusive, and in specific cases can provide relief of obstructive symptoms. Two of our cases occurred more than 15 years ago, and previous histological diagnosis was not obtained in any of the three patients with lymphoma. In the patient who survived, resection was considered complete (histological analysis did not specify whether the margins were negative or positive) and adjuvant therapy was successful in controlling the disease; the patient had been in remission for 206 months. In the other two cases, resection was partial and adjuvant chemotherapy failed to control the disease, both patients surviving less than three months.
The other patient in whom surgery was curative had an epithelioid hemangioendothelioma and had no signs of recurrence after 92 months. This is a rare malignant vascular tumor, with potential for systemic metastasis, and resection is accordingly indicated to prevent its spread.10
Finally, although adjuvant therapy appears to have been successful in one lymphoma patient, it was not curative in any of the sarcoma patients, though it may have increased survival and improved quality of life. However, the small size of our series makes it almost impossible to assess the efficacy of these therapeutic approaches.
ConclusionIn this study, patients with primary malignant cardiac tumors had a low survival rate. The surgery was rarely curative, but there was no mortality due to intraoperative complications and the incidence of surgical morbidity was low. In the sarcoma group, complete resection significantly increased survival, but even partial resection provided relief of obstructive symptoms and improved quality of life.
Due to the rarity of such tumors, a multicenter database could improve knowledge and help clarify indications for cardiac surgery as a treatment option.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Saraiva J, Antunes PE, Carvalho L, Antunes MJ. Tumores cardíacos primários malignos: resultados cirúrgicos. Rev Port Cardiol. 2016;35:199–204.