Today's healthcare policies rely heavily on data that has been gathered from multiple small studies in intrinsically varied populations. We sought to describe the prevalence, comorbidities and outcomes of atrial fibrillation (AF) in the population of a specific region where all healthcare centers have implemented a common information technology (IT) structure.
MethodsThe total number of inhabitants was obtained from the healthcare area's IT system. Information pertaining to AF was derived from various datasets in the data warehouse of the Galician regional health service.
ResultsIn the healthcare area of Santiago de Compostela (n=383000), the diagnosis of AF was coded in 7990 (2.08%) individuals in 2013. Mean age was 76.83±10.5 years, mean CHA2DS2-VASc score was 3.5, 4056 (50.8%) were female and 72.6% were receiving oral anticoagulants. Up until December 31, 2015, 1361 patients died from all causes (17%), 478 (6%) of them in-hospital, with 30 deaths secondary to intracranial bleeding (0.4%) and 125 to stroke (1.6%). On multivariate analysis, age, gender, heart failure, diabetes, previous thromboembolic events and dementia were independently associated with all-cause mortality. Similarly, age, gender and previous thromboembolic events were associated with future thromboembolic events. Oral anticoagulation was found to be protective against mortality and thromboembolic events.
ConclusionsIn this study, we report for the first time the true prevalence of diagnosed AF and its clinical characteristics, treatment and prognosis in a Spanish healthcare area, based on the systematic integration of data available from a universally adopted health IT system within the region.
As políticas dos cuidados de saúde confiam essencialmente em dados provenientes de múltiplos pequenos estudos no âmbito de populações diversificadas. Desejamos descrever a prevalência, as comorbilidades e os resultados da fibrilhação auricular (FA) numa população pertencente a uma área específica onde os centros dos cuidados de saúde implementaram uma estrutura informática comum.
MétodosO número total de habitantes foi obtido através do sistema informático das áreas dos cuidados de saúde. A informação relativa à FA provém de diversos conjuntos de dados proveniente do banco de dados do Serviço Saúde da Galiza.
ResultadosNa área dos cuidados de saúde de Santiago de Compostela (n = 348 985), ao longo de 2013, o diagnóstico de FA foi codificado em 7990 (2,08%) indivíduos. A idade média foi 76,83 ± 10,5 anos, valor médio CHA2DS2-VASc = 3,5, sendo 4056 (50,8%) mulheres, 72,6% das quais administradas com anticoagulantes orais. Até 31 de dezembro de 2015, 1361 doentes morreram de mortalidade geral (17%). Destes, 478 (6%) estavam internados, tendo sido registadas 30 mortes secundárias a hemorragia intracraneana (0,4%) e 125 acidentes vasculares cerebrais (1,6%). Numa análise multivariável, a idade, o sexo, a insuficiência cardíaca, a diabetes, os eventos tromboembólicos e a demência foram associados independentemente da mortalidade em geral. Do mesmo modo, a idade, o sexo e o(s) tromboembólico(s) prévio(s) foram associados à ocorrência de futuros eventos tromboembólicos. Considerou-se que a anticoagulação oral teve um efeito protetor na mortalidade e nos eventos tromboembólicos.
ConclusõesNeste estudo, apresentamos pela primeira vez a prevalência real da FA diagnosticada, com as características clínicas, tratamento e prognóstico de uma área específica de cuidados de saúde em Espanha, em conformidade com a integração sistemática dos dados disponíveis num processamento informático sanitário adotado na região.
Processing large data volumes, structured or unstructured, using ‘big data’ technology, has great potential waiting to be realized in the areas of prevention, research, personalization of treatment and management of integrated health services. One trend of increasing importance in the healthcare sector is working with sets of limited data, which are then pooled and combined for specific purposes, which can improve the end results. In the Galician regional health service (Servicio Gallego de Salud, SERGAS), information technology (IT) systems are used to manage data for the whole population, which are recorded in data repositories that store patients’ electronic clinical records. This is particularly useful for highly prevalent diseases such as atrial fibrillation (AF), as these can then be reliably identified using specific codes.
It is essential to know the prevalence, distribution and course of AF, since this arrhythmia is associated with high morbidity and mortality.1,2 However, no data are available on its epidemiology or overall trends in Spain based on real populations, since most research on this disease is based on retrospective and/or prospective registry studies, in which the populations are often randomly selected and most of which are cross-sectional in design, which inevitably risks screening bias. Therefore, these studies can only estimate its prevalence.3–11
The purpose of this study is to characterize the population of AF patients in terms of clinical characteristics, treatment and prognosis in a region in which electronic clinical records have been implemented in 100% of centers, combining different data sources to determine prevalence, comorbidities, concomitant medications and prognostic indicators, as well as to suggest new healthcare policies for this highly prevalent cardiac disease.
MethodsStudy populationThe total number of the assigned subjects in our area was obtained from the population information system of the Galician regional health service.12 Information on AF patients was obtained from various sources within the data warehouse of the Galician regional health service, using analytical tools (Sistemas de Información de Análisis Complejo, SIAC) to retrieve standardized and structured data on primary healthcare (SIAC-AP), hospital discharges (SIAC-HA), pharmaceutical prescriptions (SIAC-PF), and patient characteristics (SIAC-CID). Clinical coding was based on international classifications: International Classification of Primary Care, version 2 (ICPC-2-E), International Classification of Diseases (ICD), 9th revision, Clinical Modification (ICD-9-CM) and the Anatomical Therapeutic Chemical (ATC) Classification System. A qualified expert searched for specific data from different modules and matched them with an active episode of AF for each patient, using a personal identification code (PIC).
Patient selection and identification, calculation of the CHA2DS2-VASc score, and medicationICPC-2-E (cut-off date: 21/12/2013):
Patients with an episode with the ICPC-2-E code K78 (atrial fibrillation/flutter) (SIAC-AP) were selected.
Patients with an episode with code K83 (valve disease) (SIAC-AP) were identified.
The CHA2DS2-VASc score was calculated as stipulated in the European Society of Cardiology guidelines.13 This scale awards 1 point for each of the following risk factors: heart failure, hypertension, age 65-74 years, vascular disease (myocardial infarction, peripheral artery disease or aortic plaque), female gender and diabetes, and 2 points for a previous stroke and age 75 years or older. Female gender was considered a risk factor when another CHA2DS2-VASc component was present. The components of the score were coded as follows:
- –
History of acute congestive heart failure with one of the following codes: K74 (ischemic heart disease with angina), K75 (acute myocardial infarction), K76 (ischemic heart disease without angina), K77 (heart failure) (SIAC-AP).
- –
Hypertension: K85 (elevated blood pressure), K86 (hypertension, uncomplicated), K87 (hypertension, complicated) (target organ damage), K85 (elevated blood pressure) (SIAC-AP).
- –
Age 75 years or older and gender (SIAC-CID).
- –
Diabetes: T89 (diabetes, insulin-dependent), T90 (diabetes, non-insulin-dependent) (SIAC-AP).
- –
Stroke or acute transient ischemia symptoms: K89 (transient cerebral ischemia), K90 (stroke/cerebrovascular accident), K91 (cerebrovascular disease), K92 (atherosclerosis/peripheral vascular disease), K93 (pulmonary embolism) (SIAC-AP).
Patients with active prescriptions for oral anticoagulation (OAC) or antiplatelets on 21/12/2013 were identified (SIAC-PF).
The following drugs are identified with an ATC code: aspirin and/or clopidogrel, acenocoumarol, warfarin (with calculation of defined daily dose), apixaban, dabigatran and rivaroxaban.
Complications (January 1, 2014 to December 31, 2015)The minimum basic data set (MBDS) is a set of variables obtained at the time of hospital discharge, mandatory in all public health system hospitals in Spain, consisting of administrative, clinical and demographic data and providing information on the patient, his/her environment, the healthcare-providing institution and the hospital attendance process. The clinical variables (primary diagnosis, other diagnosis, surgical procedure and other therapeutic or diagnostic procedures) are the most useful instruments for assessment of clinical activity and are coded according to the ICD-9-CM.
Therapeutic failure and the occurrence of adverse effects were identified from primary and secondary diagnoses in the MBDS related to thromboembolic events (ischemic stroke, deep venous thrombosis and pulmonary embolism) and bleeding events (gastrointestinal, retroperitoneal, hematuria, nervous system and unspecified) and their ICD (8th and 9th revisions) codes.
Causes of death were divided into inpatient and outpatient deaths. Based on the codes assigned to the primary cause of death, deaths were also classified by a single investigator (MRM) as cardiovascular death (myocardial infarction, heart failure, arrhythmia, endocarditis, embolism or bleeding) or non-cardiovascular death (infection, malignancy, trauma, gastrointestinal, kidney failure, respiratory failure, hematological, rhabdomyolysis, diabetes, neurological disorders, vascular failure, or gynecological).
Statistical analysisIn an initial descriptive analysis, Gaussian continuous variables were expressed as mean and standard deviation and non-Gaussian continuous variables as median (minimum and maximum). To identify differences between males and females, the parametric ANOVA test was used for Gaussian variables and the Kruskal-Wallis test for non-Gaussian variables. Categorical variables were compared using the chi-square test. All-cause mortality and cardiovascular mortality were calculated using Cox regression models (including the variables age, gender, heart failure, hypertension, previous thromboembolic events, vascular disease, diabetes, valvular AF, dementia, OAC and antiplatelet therapy).
The quantitative variables assessed were age, gender, valvular and non-valvular AF, peripheral artery disease, diabetes (insulin-dependent or non-insulin-dependent), hypertension, pulmonary thromboembolism, transient ischemic attack, heart failure, myocardial infarction, medication with warfarin/acenocoumarol, aspirin, clopidogrel, rivaroxaban, apixaban or dabigatran, and death (inpatient and outpatient). Quantitative variables were age and CHA2DS2-VASc score.
A two-sided test was used to calculate p values, and values <0.05 were considered statistically significant. All statistical calculations were performed using IBM SPSS version 19.
ResultsIn the healthcare area of Santiago de Compostela (383000 subjects), the diagnosis of AF (paroxysmal or persistent) was coded in 7990 (2.08%) individuals. In individuals aged 0-14 years there were 53684 subjects and 0 AF cases (0%), in those aged 14-64 years there were 956 cases of AF (0.38%), and in those aged >64 years there were 91052 cases (7.72%). Of these 7990 patients, 846 cases were valvular AF and the remainder (7144; 89.4%) were non-valvular AF (Table 1).
Distribution of clinical profiles according to gender.
| Males (n=3934; 49.2%) | Females (n=4056; 50.8%) | Total (n=7990) | p | |
|---|---|---|---|---|
| Age, years (SD) | 74.42 (11.18) | 79.17 (9.23) | 76.83 (10.51) | 0.000 |
| Age <65 years, n (%) | 681 (17.3) | 275 (6.7) | 956 (12.0) | 0.000 |
| Heart failure, n (%) | 1151 (29.3) | 1025 (25.3) | 2176 (27.2) | 0.000 |
| Hypertension, n (%) | 2572 (65.4) | 3020 (74.5) | 5592 (70.0) | 0.000 |
| Diabetes, n (%) | 975 (24.8) | 906 (22.3) | 1881 (23.5) | 0.010 |
| Vascular disease, n (%) | 301 (7.7) | 209 (5.2) | 510 (6.4) | 0.000 |
| Previous stroke, n (%) | 353 (9) | 381 (9.4) | 734 (9.2) | 0.515 |
| Valvular AF, n (%) | 379 (9.6) | 467 (11.7) | 846 (10.6) | 0.006 |
| Dementia, n (%) | 76 (1.9) | 211 (5.2) | 287 (3.6) | 0.000 |
| Antiplatelet therapy, n (%) | 515 (13.1) | 532 (13.1) | 1047 (13.1) | 0.973 |
| OAC, n (%) | 2825 (71.8) | 2974 (73.3) | 5799 (72.6) | 0.129 |
| CHA2DS2-VASc score <2 | 682 (17.3) | 127 (3.1) | 809 (10.1) | 0.000 |
AF: atrial fibrillation; OAC: oral anticoagulation; SD: standard deviation.
The mean age of the study population was 76.83±10.51 years and 4056 patients (50.8%) were female. The mean CHA2DS2-VASc score was 3.5±1.54. The distribution of the score was 0 points in 285 subjects (3.6%), 1 in 524 (6.6%), 2 in 1106 (13.8%), 3 in 1940 (24.3%), 4 in 2163 (27.1%), 5 in 1280 (16.0%), 6 in 503 (6.3%), 7 in 150 (1.9%), 8 in 38 (0.5%) and 9 in one (0.1%) (Table 2). By score component, the most frequently coded variable was hypertension (5992; 70%) and the least frequently coded was vascular disease (510; 6.4%). For patients scoring only 1 point on the CHA2DS2-VASc scale, this point referred to the following components, in order of decreasing frequency: age 65-75 (n=185, 35.3%); hypertension (n=152; 29%); female gender (n=127; 24.2%); heart failure (n=30; 5.7%); diabetes (n=26; 5.0%); and vascular disease (n=4; 0.8%).
Percentages of oral anticoagulation and event rates according to CHA2DS2-VASc score.
| CHA2DS2-VASc score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| OAC | (n, %) | (285, 3.6) | (524, 6.6) | (1106, 13.8) | (1940, 24.3) | (2163, 27.1) | (1280, 16.0) | (503, 6.3) | (150, 1.9) | (38, 0.5) | (1, 0.0) | Total (7990, 100) |
| No, n (%) | 183 (64.2) | 227 (43.3) | 318 (28.8) | 488 (25.2) | 511 (23.6) | 285 (22.3) | 128 (25.4) | 41 (27.3) | 10 (26.3) | 0 (0.0) | 2191 (27.4) | |
| Yes, n (%) | 102 (35.8) | 297 (56.7) | 788 (71.2) | 1452 (74.8) | 1652 (76.4) | 995 (77.7) | 375 (74.6) | 109 (72.7) | 28 (73.7) | 1 (100) | 5799 (72.6) | |
| Stroke, n (%) | 0 (0) | 3 (0.6) | 11 (1) | 38 (2) | 24 (1.1) | 23(1.8) | 22 (4.4) | 3 (2.0) | 1 (2.6) | 0 (0) | 125 (1.6) | |
| Bleeding events, n (%) | 0 (0) | 3 (0.6) | 13 (1.2) | 49 (2.5) | 51 (2.4) | 28 (2.2) | 19 (3.8) | 1 (0.7) | 0 (0) | 0 (0) | 164 (2.1) | |
| Intracranial bleeding, n (%) | 0 (0) | 0 (0) | 3 (0.3) | 11 (0.6) | 8 (0.4) | 3 (0.2) | 5 (1) | 0 (0) | 0 (0) | 0 (0) | 30 (0.4) | |
| All-cause mortality, n (%) | 6 (2.1) | 22 (4.2) | 105 (9.5) | 291 (15.0) | 420 (19.4) | 313 (24.5) | 147 (29.2) | 39 (26) | 17 (44.7) | 1 (100) | 1361 (17) | |
OAC: oral anticoagulation.
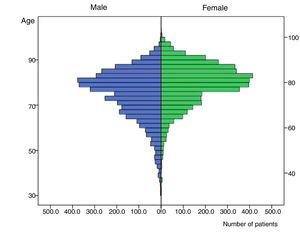
The distribution of CHA2DS2-VASc components varied between genders, with males presenting heart failure and hypertension less frequently but diabetes, vascular disease and previous thromboembolic events more frequently than females (Table 1). Moreover, males tended to develop AF at an earlier age than females (74.42±11.18 vs. 79.17±9.23 years; p<0.001). Figure 1 shows an age/gender distribution histogram. For example, the proportion of patients younger than 65 years old (n=956; 12%) varied significantly between genders: 681 (8.5%) men vs. 275 women (3.4%) (p<0.0001).
Within this population, 5799 patients (72.6%) were on OAC: 679 (8.5%) on warfarin, 5040 (63.1%) on acenocoumarol, six (0.07%) on apixaban, 383 (4.8%) on dabigatran and 50 (0.6%) on rivaroxaban, and 1060 (13.3%) were on antiplatelets: 964 (12.1%) on aspirin and 96 (1.2%) on clopidogrel.
Of patients with CHA2DS2-VASc scores ≥2, 5400 (75.2%) were anticoagulated (1782 without OAC, 24.8%), while of patients with CHA2DS2-VASc score <2 (n=809), 399 (49.3%) had been prescribed some kind of OAC. Dual therapy (combination of an OAC plus antiplatelet) was recorded in 154 (1.9%) patients.
Among patients with CHA2DS2-VASc score of 1, 39 subjects were on OAC (49.3%). Table 3 shows proportions of OAC prescription. Although the number of patients is relatively low, there appears to be a greater tendency to prescribe OAC when the risk factor was age, diabetes or heart failure; similar prescribing levels for hypertension and vascular disease; and less likelihood of OAC prescription when the risk factor was female gender.
Percentages of oral anticoagulation prescription in patients with a single CHA2DS2-VASc risk factor.
| No OAC (n=226; 43.1%) | OAC (n=298; 56.9%) | |
|---|---|---|
| Female gender | 74 (32.7%) | 53 (17.8%) |
| Age 65-74 years | 59 (26.1%) | 126 (42.3%) |
| Vascular disease | 2 (0.9%) | 2 (0.7%) |
| Diabetes | 9 (4%) | 17 (5.7%) |
| Hypertension | 73 (32.3%) | 79 (26.5%) |
| Heart failure | 9 (4%) | 21 (7%) |
OAC: oral anticoagulation.
As of December 31, 2015, 1361 patients (17%) had died (all-cause mortality), 478 of them inpatients (6% of the total) and the rest outpatients. For inpatient deaths, a diagnostic code was assigned by primary cause of admission, and this cause was cardiovascular for 175 subjects (36.6%) and non-cardiovascular for the remainder (n=303, 63.4%). Table 4 shows the code of the primary cause of hospital admission for patients who died. Thirty-eight subjects (7.9%) had a bleeding event, while 30 (0.4%) subjects had intracranial bleeding. Of these 30 patients, 25 were on OAC, three were on antiplatelets and two were not receiving either. All these events occurred in patients with CHA2DS2-VASc scores ≥2 (Table 2).
Main causes of death of patients with atrial fibrillation who died during hospital stay.
| Cause of death, n (%) | 478 (6) |
|---|---|
| Endocarditis | 1 (0.2) |
| Myocardial infarction | 3 (0.6) |
| Heart failure | 69 (14.4) |
| Arrhythmias | 4 (0.8) |
| Embolic events | 39 (8.2) |
| Bleeding events | 38 (7.9) |
| Infection | 97 (20.3) |
| Malignancy | 38 (7.9) |
| Trauma | 17 (3.6) |
| Gastrointestinal | 32 (6.7) |
| Renal disease | 8 (1.7) |
| Respiratory disease | 100 (20.9) |
| Hematological disease | 2 (0.4) |
| Aortic disease | 1 (0.2) |
| Rhabdomyolysis | 1 (0.2) |
| Diabetes | 1 (0.2) |
| Neurological disease | 5 (1) |
| Adverse drug effect | 1 (0.2) |
| Valvular insufficiency | 20 (4.2) |
| Gynecological disease | 1 (0.2) |
Differences in overall mortality were observed between genders (Table 5). As for overall mortality, in the multivariate analysis the variables age, gender, heart failure, diabetes, previous thromboembolic event, dementia, oral anticoagulation and antiplatelet therapy were independent predictive factors for all-cause mortality (Table 6). However, valvular AF, hypertension and vascular disease were not significant.
Event rates according to gender.
| Males (n=3934; 49.2%) | Females (n=4056; 50.8%) | Total (n=7990) | p | |
|---|---|---|---|---|
| Intracranial bleeding | 14 (0.4%) | 16 (0.4%) | 30 (0.4%) | 0.778 |
| Bleeding events | 86 (2.2%) | 78 (1.9%) | 164 (2.1%) | 0.407 |
| Stroke | 44 (1.1%) | 81 (2.0%) | 125 (1.6%) | 0.002 |
| In-hospital death | 235 (6.0%) | 243 (6.0%) | 478 (6.0%) | 0.974 |
| All-cause mortality | 617 (15.7%) | 744 (18.3%) | 1361 (17.0%) | 0.002 |
Univariate and multivariate Cox regression analyses for all-cause mortality and stroke in the overall population.
| All-cause mortality | Stroke | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| Age | 1.11 | 1.10-1.12 | 0.000 | 1.10 | 1.09-1.11 | 0.000 | 1.05 | 1.03-1.07 | 0.000 | 1.04 | 1.02-1.06 | 0.000 |
| Female gender | 1.21 | 1.07-1.36 | 0.002 | 0.83 | 0.74-0.95 | 0.007 | 1.80 | 1.24-2.60 | 0.002 | 1.51 | 1.03-2.20 | 0.03 |
| Heart failure | 2.00 | 1.77-2.26 | 0.000 | 1.63 | 1.43-1.87 | 0.000 | 1.08 | 0.73-1.59 | 0.692 | |||
| Hypertension | 1.18 | 1.03-1.34 | 0.013 | 0.93 | 0.81-1.07 | 0.33 | 0.88 | 0.60-1.27 | 0.87 | |||
| Thromboembolic event | 1.73 | 1.44-2.06 | 0.000 | 1.30 | 1.08-1.58 | 0.007 | 2.27 | 1.43-3.59 | 0.000 | 1.95 | 1.22-3.10 | 0.005 |
| Vascular disease | 1.39 | 1.11-1.73 | 0.003 | 1.17 | 0.93-1.48 | 0.18 | 1.14 | 0.57-2.26 | 0.707 | |||
| Diabetes | 1.35 | 1.18-1.54 | 0.000 | 1.37 | 1.18-1.57 | 0.000 | 0.89 | 0.58-1.37 | 0.606 | |||
| Valvular AF | 4.69 | 3.69-5.96 | 0.000 | 1.18 | 0.97-1.44 | 0,09 | 1.34 | 0.79-2.24 | 0.272 | |||
| Dementia | 4.69 | 3.69-5.96 | 0.000 | 2.87 | 2.22-3.72 | 0.000 | 1.12 | 0.45-2.76 | 0.805 | |||
| OAC | 0.75 | 0.66-0.86 | 0.000 | 0.69 | 0.59-0.82 | 0.000 | 0.52 | 0.36-0.75 | 0.000 | 0.64 | 0.41-0.99 | 0.04 |
| Antiplatelet therapy | 1.21 | 1.03-1.43 | 0.024 | 0.75 | 0.60-0.93 | 0.008 | 2.22 | 1.47-3.35 | 0.000 | 1.53 | 0.92-2.53 | 0.09 |
AF: atrial fibrillation; CI: confidence interval; HR: hazard ratio; OAC: oral anticoagulation.
As of December 31, 2015, 125 stroke cases had been notified (1.6%). Independent factors related to stroke were age, gender and previous thromboembolic events (Tables 5 and 6). OAC was a protective factor against cerebral thromboembolic events (hazard ratio [HR] 0.64; 95% confidence interval [CI] 0.41-0.99, p=0.04).
Finally, during this period, 164 bleeding events were coded (2.1%) (including the 38 subjects for whom it was the cause for primary hospital admission). Of these, 80.1% were on OAC and 15.5% were on antiplatelet therapy. In the multivariate model, age (HR 1.03; 95% CI 1.01-1.05, p=0.04) and valvular AF (HR 1.95; 95% CI 1.31-2.91, p=0.001) were predictive of bleeding events, while OAC showed a tendency to increase the risk, without reaching statistical significance (HR 1.44; 95% CI 0.97-2.14, p=0.06).
DiscussionIn this study we report for the first time the real prevalence, clinical characteristics, treatment and prognosis of AF within an entire Spanish healthcare area, derived from the systematic integration of data available from a universally adopted health IT system in which electronic clinical records have been implemented in 100% of centers. The availability of reliable data on prevalence, comorbidities, treatment and outcome indicators helps in devising appropriate healthcare policies for this highly prevalent disease.
Our results confirm the prevalence rates and differences between genders shown in other Spanish registries. They also corroborate the rates of ischemic and bleeding events observed in clinical trials. Moreover, they report, for the first time in our sector, the causes of inpatient death for AF patients and call attention to the high overall associated mortality. Finally, they demonstrate the value of the CHA2DS2-VASc score for risk stratification and the protective role of OAC against both all-cause mortality and thromboembolic events, as well as identifying new risk markers such as dementia.
In recent years, various studies have set out to estimate the prevalence of AF in Spain using data gathered within the ambit of primary care and based on predetermined patient subgroups. In the Val-FAAP study,11 6% of patients seen in primary care had AF. Other studies with smaller populations have also provided estimates, such as the CARDIOTENS study,3,4 in which AF was detected in 2.75% of patients seen in primary care (17.62% for specialist consultations); the figure was 4% in the Barbanza study,5 10.2% in the PREV-ICTUS study6 and 10.3% of all patients seen in primary care in the FAPRES study.7 According to the OFRECE study,9 the prevalence of AF in Spain for the total population aged 40 years or older was 4.4%. The present analysis is not restricted by age or by any other parameter, but includes the total population within our study area and the outcomes apply to the whole of our healthcare area. An additional advantage of the use of electronic clinical records is the negligible proportion of patients lost to follow-up, since all patients had subsequent consultations in one of the health centers within the healthcare area and a death would inevitably show up in the system. This is important because no data on overall mortality for patients with AF within our area were previously available. Nevertheless, it is important to stress that this technology does not permit causal relationships to be established, so AF may occur as an epiphenomenon or as a secondary event resulting from a primary process.
As for the risk profile, the mean age was also slightly higher than in the above-mentioned registries: 76.83 years for our population, 59.2 years in the OFRECE study,9 68.4 years in the CARDIOTENS study3,4 and 71.9 years in the Val-FAAP11 and PREV-ICTUS6 studies. Distribution by gender is more similar among the above-mentioned studies, around 50% in all of them (50.8% female in the present study and 52.4%, 55.3%, 47.7% and 53.6% in the OFRECE, CARDIOTENS, Val-FAAP and PREV-ICTUS studies, respectively).
It is also worth noting that hypertension is the risk factor with the highest prevalence in all studies and with the second highest prevalence (after age) in patients with a single risk factor for thromboembolic events (CHA2DS2-VASc score <2). Overall, hypertension prevalence ranged between 66% (CARDIOTENS) and 92.7% (PREV-ICTUS). The diagnosis of AF is not considered an independent risk factor for either overall mortality or thromboembolic events, probably due to the fact that it includes all cases, from mild to severe.
It should be noted that overall thromboembolic risk in the study population was remarkably high (mean CHA2DS2-VASc score 3.5), most of the patients within our area having a CHA2DS2-VASc score ≥2 and thus in principle indication for OAC. In fact only two-thirds of patients are currently on OAC. Unfortunately, since bleeding risk was not assessed in our sample using the HAS-BLED score,13 we were unable to determine whether high bleeding risk would explain the fact that OAC was not prescribed in certain patients. However, this proportion is slightly higher than reported in previous studies (60.1% in PREV-ICTUS6 and 57% in Val-FAAP11). This, together with the fact that half of the patients with CHA2DS2-VASc scores <2 were prescribed some kind of OAC, suggests that physicians’ training on OAC prescription has improved in the past few years. When studies from the last decade3,14 are compared with contemporary studies,8,11 it can be seen that the recent trend has been to prescribe OAC for patients with CHA2DS2-VASc scores <2. Furthermore, as has recently been argued, changes in anticoagulation recommendations, resulting from CHADS2 being replaced by CHA2DS2-VASc, may have played a significant role in this increase in AO prescription.14
It is interesting that significant real-world differences can be detected between the risk profiles of men and women, with onset or at least diagnosis of AF at earlier ages in men.5,8,9 This is consistent with previous studies performed in this field. However, although overall rates of stroke and all-cause mortality were higher in women, in multivariate analysis female gender appears to be a protective factor for all-cause mortality but an independent risk factor for thromboembolic events.
The findings of the present study for our study population include the following: (a) low-risk patients show a very low incidence of ischemic events and all-cause mortality; (b) the CHA2DS2-VASc score helps identify populations at risk for both all-cause mortality and thromboembolic events; (c) not all CHA2DS2-VASc components have the same discriminatory power; (d) OAC is a protective factor for both all-cause mortality and thromboembolic events; and (e) stroke and bleeding rates in our population are even lower than those of recent clinical studies. The last finding should be treated with caution, since most of the information available today derives from studies performed with the new oral anticoagulants. The XANTUS study,15 for instance, which analyzed the use of rivaroxaban in a large population of patients with non-valvular AF (n=6784, one year of follow-up), showed similar rates of major bleeding (128 of 6784 patients, incidence 2.1 per 100 patient-years), intracranial bleeding (26, incidence 0.4) and stroke (51, incidence 0.7). However, probably due to the unselected nature of the population, there was a clear difference in all-cause mortality in our study (1361 vs. 118 patients in the XANTUS study). Our study enables us to provide detailed data on the causes of death for AF patients who died in hospital: Table 4 shows the considerable variety of both cardiovascular and non-cardiovascular causes.
Surprisingly, dementia proved to be an independent marker for overall mortality. Although its association with overall mortality in elderly patients is known,16–18 this had not previously been reported for the subgroup of patients with AF, and is thus not currently included in risk stratification scores. In our opinion, this result should be confirmed by further studies, considering its high added risk for all-cause mortality (HR=2.87, 95% CI: 2.22-3.72; p<0.001), even higher than for those of the independent CHA2DS2-VASc components.
It should be stressed that the methodology described here can be used to discover the real prevalence of a disease and can subsequently be applied to the development of planning policies. The term ‘big data’19 was coined to refer to datasets that are so large and complex that they would be very difficult to process using conventional database management tools. One of the specific challenges is how to access, distribute and use the vast amount of data, which may structured or unstructured, stored in electronic medical records. We believe that analysis of structured data available in healthcare IT systems will provide information relevant to clinical assessments. An additional advantage is that, unlike cross-sectional observational studies, this type of integration enables thorough patient follow-up, which is essential for the implementation of preventive and/or treatment policies. Likewise, the management of this type of information could be usefully applied to other diseases with a high prevalence that already have specific codes within current health systems.
LimitationsThe most important limitation of this study is the fact that the codes used are entered by multiple physicians and are not reviewed by an independent observer, which could lead to errors in coding a specific disease for a patient. However, given that AF is frequently encountered in emergency departments, primary care and other medical specialties, we believe that the proportion of wrongly coded patients is low and would therefore not alter the study's conclusions. It should, nevertheless, be noted that AF is coded without distinguishing between paroxysmal, persistent or permanent forms. However, the current evidence points to similar tendencies for thromboembolic events, heart failure and clinical management of OAC,2 making this lack of distinction less important for the purposes of this study.
Heart failure was defined based on clinical symptoms and not on left ventricular ejection fraction, a parameter known as an established risk factor for the development of thromboembolic events.13 Thus, if this variable had been taken into account, the mean overall CHA2DS2-VASc score could be expected to have been slightly higher than that reported here.
Although in our study we compare the prevalence of AF in our population with that in previous studies, it should be pointed out that most studies assessing AF prevalence are not strictly comparable due to differences in diagnostic criteria and in the study populations. We believe that the results shown here are applicable to the rest of Spain, but not necessarily to other populations with different healthcare systems or different clinical profiles.
ConclusionsIn this study, we report for the first time the prevalence, clinical characteristics, treatment and prognosis of AF derived from the systematic integration of data from a universally adopted health IT system containing information on the entire population of a region of Spain. This is the largest AF population studied in Spain to date, and the study confirms the prevalence reported in previous registries in Spain. This analysis also enabled us to record the cause of inpatient deaths for AF patients, and calls attention to the high overall associated mortality. Moreover, it supports the value of the CHA2DS2-VASc score as a tool for risk stratification and the protective role of OAC against both all-cause mortality and thromboembolic events, as well as identifying new risk indicators such as dementia.
A ‘big data’ application would allow us to organize AF information effectively and obtain process indicators and outcomes that could be available on a periodic basis. At present this type of information can only be obtained from specific analyses that are expensive, although the data could be very useful in adapting healthcare policies to the challenges posed by highly prevalent cardiac diseases.
FundingNone.
Conflicts of interestThe authors have no conflicts of interest to declare.