Therapeutic hypothermia (TH) is recommended for patients with return of spontaneous circulation (ROSC) after cardiac arrest (CA). There is still uncertainty about management, target temperature and duration of TH. In the present study we aim to describe the initial experience of a non-tertiary care center with TH after CA and to determine predictors of mortality.
MethodsDuring the period 2011-2014, out of 2279 patients hospitalized in the intensive care unit, 82 had a diagnosis of CA with ROSC. We determined predictors of mortality and neurological outcome in comatose patients with ROSC after CA treated by TH.
ResultsA total of 15 patients were included, mean age 47.3±14 years, 10 (67.0%) male. CA occurred out-of-hospital (n=11; 73.3%) or in-hospital (n=4; 26.7%), in initial shockable (n=10; 66.7%) or non-shockable (n=5, 33.3%) rhythm. The mean time from CA to ROSC (CA-ROSC) was 44.7±36.5 min. All patients met the 24-hour TH target temperature of 33°C. The mean neuron-specific enolase (NSE) level was 93.7±109.0 μg/l. Seven patients (46.7%) were discharged with good cerebral performance and eight (53.3%) died. Patients who survived had lower median age (p=0.032), shorter CA-ROSC (p=0.048), lower NSE levels (p=0.020) and initial ventricular fibrillation rhythm (p=NS).
ConclusionsThe effectiveness of TH appears to be related to younger age, shockable initial rhythm and shorter CA-ROSC time. This results indicates some lines of inquiry that should be developed in appropriate prospective studies. The role of biomarkers as predictors of prognosis is an open question, with NSE potentially playing an important role.
A Hipotermia Terapêutica (HT) é recomendada em doentes com recuperação da circulação espontânea (ROSC) após paragem cardíaca (PC). Dúvidas persistem sobre a melhor abordagem, a temperatura alvo e a duração da técnica. Neste estudo descrevemos a experiência inicial de um centro não-terciário com HT após PC e procuramos preditores de mortalidade.
MétodosDurante os anos de 2011-2014, dos 2279 doentes hospitalizados na unidade de cuidados intensivos, 68 tinham diagnóstico de PC com ROSC. Determinámos preditores de mortalidade e prognóstico neurológico nos doentes comatosos com ROSC após PC, submetidos a HT.
ResultadosQuinze doentes, 47,3±14 anos, dez (67,0%) do sexo masculino. A PC ocorreu extra (n=11; 73,3%) ou intra-hospitalar (n=4; 26,7%), em ritmo cardíaco inicial desfibrilhável (n=10; 66,7%) ou não-desfibrilhável (n=5, 33,3%). O tempo médio decorrido desde a PC até à ROSC (PC-ROSC) foi de 44,7±36,5 min. O valor médio da enolase específica dos neurónios (NSE) foi de 93,7±109,0 μg/L. Sete doentes (46,7%) tiveram alta hospitalar com bom desempenho cerebral e oito doentes (53,3%) faleceram. Os doentes que sobreviveram apresentavam idade média inferior (p=0,032), menor tempo PC-ROSC (p=0,048), doseamentos mais baixos de NSE (p=0,020) e ritmo inicial predominante de fibrilhação ventricular (p=NS).
ConclusõesA eficácia da HT parece relacionada com idades mais jovens, ritmo inicial desfibrilhável e tempo PC-ROSC reduzido. Estes resultados apontam algumas linhas de investigação que estudos prospetivos adequados deverão desenvolver. O papel de biomarcadores preditores de prognóstico é um tema em aberto, devendo a NSE ocupar um lugar particular neste domínio.
advanced life support
B-type natriuretic peptide
cardiac arrest
Cerebral Performance Category
cardiopulmonary resuscitation
computed tomography
cardiovascular
electroencephalography
Glasgow Coma Scale
intensive care unit
International Liaison Committee on Resuscitation
neuron-specific enolase
N-terminal pro-B-type natriuretic peptide
percutaneous coronary intervention
return of spontaneous circulation
ST-segment elevation myocardial infarction
therapeutic hypothermia
time from collapse to cardiopulmonary resuscitation
time from collapse to return of spontaneous circulation
time from collapse to beginning of therapeutic hypothermia
transthoracic echocardiography
ventricular fibrillation
Cardiac arrest (CA) with cerebral ischemia can lead to severe neurological damage and death. The survival rate of out-of-hospital CA is reported to be 2-11%, rising to 20-40% in witnessed ventricular fibrillation (VF) cases.1–3
Induced hypothermia has been employed as a therapeutic approach for CA survivors and other critically ill patients for decades.4 Two randomized controlled trials published in 2002 reported a dramatic improvement in survival and neurological outcome for comatose survivors of out-of-hospital VF CA with therapeutic hypothermia (TH).5,6
TH (32°C-34°C for 12-24 hours) has been the standard of care for patients remaining comatose after resuscitation.7,8 A recent randomized trial found a similar outcome in patients treated with targeted temperature management at either 33°C or 36°C,9 prompting the International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support (ALS) Task Force to perform a systematic review.10 Currently, a constant temperature between 32°C and 36°C for at least 24 hours is the recommended targeted temperature management for adults following out-of-hospital CA with an initial shockable rhythm and, with a lower level of evidence, for out-of-hospital CA with a non-shockable rhythm and in-hospital CA.11
The aim of this retrospective analysis was to describe the initial experience of a non-tertiary care center and to determine predictors of mortality and neurological outcome at discharge for CA patients treated with TH, focusing on a combination of initial rhythm, time to return of spontaneous circulation (ROSC) and laboratory biomarkers.
MethodsDesign and settingWe performed a retrospective review of consecutive patients who suffered CA admitted between January 1, 2011 and December 31, 2014 to the intensive care unit (ICU) at a non-tertiary care center.
Inclusion and exclusion criteriaWe studied comatose adults (unable to follow verbal commands after ROSC) aged ≥18 years admitted to the ICU with ROSC after in-hospital or out-of-hospital CA who were treated by TH. Patients without known time of CA, in a comatose state before CA, or with a terminal illness that preceded CA were excluded.
Therapeutic hypothermia techniquePatients treated by TH were cooled to a target temperature of 33°C with a combination of core-cooling and surface-cooling methods. By protocol 30 ml of cold (4°C) lactated Ringer's solution per kg was administered intravenously over 30 min. An external cooling device, Arctic Sun®, was also used. This is a targeted temperature management system that uses chilled water circulating in gel-coated pads to maintain body temperature in the desired range. An esophageal probe was used to monitor core temperature.
All patients underwent three phases of TH: a fast induction phase to 33°C; a maintenance phase for 24 hours at 33°C; and a rewarming phase of about 0.25°C of warming per hour until 36°C.
Data collection and definitionsThe following baseline characteristics were collected from patients’ medical records: demographic data; cardiovascular risk factors; place and initial rhythm of CA; time from collapse to cardiopulmonary resuscitation (CPR), to ROSC and to TH; admission data including Glasgow Coma Scale (GCS) score and laboratory tests including levels of neuron-specific enolase (NSE), the gamma isomer of enolase expressed mainly in neurons and neuroectodermal cells, measured within 72 hours of CA.
Bedside focused transthoracic echocardiography (TTE) and cranial computed tomography (CT) were performed within 24 hours of admission. Coronary angiography was performed before TH. Etiological diagnosis of CA was determined according to the current European Society of Cardiology guidelines.12–15
CA was defined as receipt of chest compressions for pulselessness as determined by a healthcare provider. The first monitored rhythm was recorded as the initial rhythm of CA.
An initial neurological assessment and GCS score at admission were obtained, in order to identify comatose patients.
Outcome measuresIn-hospital mortality and Cerebral Performance Category (CPC) were assessed at ICU discharge, hospital discharge and at six months after discharge. Adverse events and length of ICU and hospital stay were assessed. Statistical analyses were performed in order to determine predictors of outcome.
CPC categories were defined according to Safar16 as follows: CPC 1 – good cerebral performance; CPC 2 – moderate cerebral disability; CPC 3 – severe cerebral disability; CPC 4 – coma or vegetative state; CPC 5 – brain death. CPC was scored based on information from hospital charts and follow-up notes.
Adverse events reported were defined as major bleeding (any intracranial bleeding or clinically overt signs of bleeding associated with a drop in hemoglobin of ≥5 g/dl or hematocrit of ≥15%, or fatal bleeding – a bleeding event that directly led to death within seven days), non-major bleeding (any clinically overt sign of bleeding associated with a fall in hemoglobin of 2 to <5 g/dl or hematocrit of 6 to <15%), seizures, arrhythmias (any documented abnormal rhythm that did not precipitate a re-arrest) and re-arrest. Isolated microorganisms and electrolyte and metabolic disturbances were also analyzed.
Statistical analysisThe statistical analysis was performed using SPSS® software for Windows, version 22.0. Categorical variables were expressed as a percentage of the total sample and compared using the chi-square test or Fisher's exact test, as appropriate. Continuous variables were expressed as mean ± standard deviation (SD); the Student's t test was used to compare normally distributed variables and the Mann-Whitney U test to compare variables without a normal distribution. Cut-off values were determined for each predictor for death and odds ratios (OR) and 95% confidence intervals (CI) were calculated. The data analysis was performed with a significance level of p<0.05.
ResultsPatient populationBetween January 1, 2011 and December 31, 2014, 2279 patients were hospitalized in the ICU, of whom 68 had ROSC after CA (46 male, age 61.0±18.8 years). In accordance with the inclusion and exclusion criteria, a total of 15 patients were enrolled in the study. Mean age was 47.3±14.3 years and 10 (66.6%) were male. In 10 patients (66.6%) at least one cardiovascular risk factor was present. The patients’ baseline characteristics are shown in Table 1.
Baseline characteristics of the study population.
| Characteristics, n (%) or mean ± SD | Study population (n=15) |
|---|---|
| Gender | |
| Male | 10 (66.6%) |
| Female | 5 (33.3%) |
| Age, years | 47.3±14.3 |
| CV risk factors | |
| Hypertension | 6 (40.0%) |
| Diabetes | 3 (20.0%) |
| Dyslipidemia | 5 (33.3%) |
| Active smoking | 6 (40.0%) |
| BMI ≥25 kg/m2 | 2 (13.3%) |
| Location of cardiac arrest | |
| Out-of-hospital | 11 (73.3%) |
| In-hospital | 4 (26.7%) |
| VF rhythm at presentation | 10 (66.7%) |
| Asystole rhythm at presentation | 5 (33.3%) |
| CA-CPR, min | 14.3±12.4 |
| CA-ROSC, min | 44.7±36.5 |
| CA-TH, min | 342±186 |
| GCS | 5.1±2.9 |
| Laboratory values | |
| Hemoglobin, g/dla | 13.5±2.3 |
| Sodium, mmol/la | 139.9±5.5 |
| Potassium, mmol/la | 3.8±1.0 |
| Total bilirubin, μmol/la | 10.9±6.9 |
| AST, U/la | 281.7±335.5 |
| ALT, U/la | 171.4±160.4 |
| NSE, μg/la | 93.7±109.0 |
| Troponin I, ng/mlb | 38.0±42.5 |
| BNP, pg/mlb | 415.0±373.0 |
| Renal function | |
| Creatinine, mg/dla | 1.19±6.9 |
| BUN, mmol/la | 7.0±3.7 |
| eGFR, ml/min/1.73 m2a | 65.3±19.2 |
| eGFR <60 ml/min/1.73 m2a | 6 (40.0%) |
| LV function | 14 (93.3%) |
| LV diastolic dimension, mm | 52±6.4 |
| LV systolic dimension, mm | 35±8.2 |
| LV fractional shortening, % | 36.8±3.3 |
| “Eyeball” LV ejection fraction <50% | 6 (42.9%) |
ALT: alanine transaminase; AST: aspartate transaminase; BMI: body mass index; BNP: B-type natriuretic peptide; BUN: blood urea nitrogen; CV: cardiovascular; eGFR: estimated glomerular filtration rate (Modification of Diet in Renal Disease formula); GCS: Glasgow Coma Scale; LV: left ventricular; NSE: neuron-specific enolase; CA-CPR: time from collapse to cardiopulmonary resuscitation; CA-ROSC: time from collapse to return of spontaneous circulation; CA-TH: time from collapse to beginning of therapeutic hypothermia; VF: ventricular fibrillation.
CA occurred out-of-hospital (n=11; 73.3%) or in-hospital (n=4; 26.7%) in initial VF (n=10; 66.7%) or asystole (n=5; 33.3%) rhythm. No ventricular tachycardia or pulseless electrical activity was reported. The mean time from CA to CPR (CA-CPR) was 14.3±12.4 min, minimum 1, maximum 40; mean time to ROSC (CA-ROSC) was 44.7±36.5 min, minimum 10, maximum 120; and mean time to TH (CA-TH) was 342±186 min, minimum 90, maximum 780. Mean GCS at admission was 5.1±2.9, median 3, 25th percentile 3, 75th percentile 8.
Bedside focused TTE was performed within 24 hours of admission in 14 patients (93.3%); eyeball left ventricular ejection fraction was estimated and was low only in the non-survival group. Cranial CT was not performed systematically (in only seven patients, 46.6%); all but one already had signs of hypoxic encephalopathy. Emergency coronary angiography was performed in 14 (93.3%) patients, all before TH. During ICU stay, all patients were under inotropic hemodynamic support.
All patients were maintained at a TH target temperature of 33°C for 24 hours. During TH, esophageal temperature, heart rate, and mean arterial blood pressure were continuously monitored and documented hourly for all patients.
The etiological diagnosis of CA, defined according to the current European Society of Cardiology guidelines,12–15 coronary angiography findings, and treatment are shown in Table 2.
Etiological diagnosis, coronary angiography findings and treatment.
| Diagnosis, n (%) | Study population (n=15) |
|---|---|
| MI | 10 (66.6%) |
| ST-elevation | 6 (40.0%) |
| Non-ST-elevation | 4 (26.7%) |
| Cardiomyopathies | 3 (20.0%) |
| ARVC | 1 (6.7%) |
| HCM | 1 (6.7%) |
| LVNC | 1 (6.7%) |
| Type 1 Brugada syndrome | 1 (6.7%) |
| Unknown diagnosisa | 1 (6.7%) |
| Treatment, n (%) | Study population (n=15) |
|---|---|
| Coronary angiography | 14 (93.3%) |
| Normal epicardial coronary arteries | 4 (28.6%) |
| Significant coronary angiographic lesions | 10 (71.4%) |
| PCI | 9 (60.0%) |
| Primary angioplasty | 6 (66.7%) |
| LAD | 5 (83.3%) |
| LCX | 1 (16.7%) |
| Non-primary angioplasty | 3 (33.3%) |
| LAD | 2 (66.7%) |
| LCX | 1 (33.3%) |
| Implanted cardioverter-defibrillator | 4 (26.7%) |
ARVC: arrhythmogenic right ventricular cardiomyopathy; HCM: hypertrophic cardiomyopathy; LAD: left anterior descending artery; LCX: circumflex artery; LVNC: left ventricular non-compaction; MI: myocardial infarction; PCI: percutaneous coronary intervention.
CPC was assessed at ICU discharge (CPC 1 or 2 in seven patients, CPC 3 in three patients, CPC 5 in five patients) and hospital discharge (CPC 1 in seven patients, CPC 5 in eight patients). In-hospital mortality occurred in eight patients (53.3%) and seven patients (46.7%) were discharged with good cerebral performance. At six months after hospital discharge, CPC scores were the same as at discharge.
Cranial CT was not performed systematically after TH or at admission. Of eight patients (53.3%) who underwent CT after TH, five had signs of hypoxic encephalopathy and all died in-hospital. Mean NSE level was 93.7±109.0 μg/l.
Length of ICU stay was 16.5±24.2 days and hospital stay was 22.5±26.4 days.
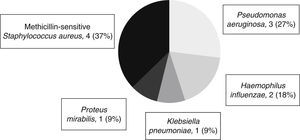
Table 3 shows the adverse events reported during the three phases of TH. As respiratory infections were frequent, occurring in 13 patients (86.7%), cultures of bronchial secretions are performed in all patients. Figure 1 shows the microorganisms isolated in bronchial secretions.
Adverse events during therapeutic hypothermia.
| Event, n (%) | Study population (n=15) |
|---|---|
| Shivering | 15 (100.0%) |
| Electrolyte disturbances | 15 (100.0%) |
| Metabolic disturbances | 15 (100.0%) |
| Hematologic disturbances | 15 (100.0%) |
| Respiratory infection | 13 (86.7%) |
| Arrhythmias | 7 (46.7%) |
| Sinus bradycardia | 5 (71.4%) |
| AFL/AF | 3 (42.9%) |
| VF/VT | 1 (14.3%) |
| Bleeding | 3 (20.0%) |
| Major | 1 (33.3%) |
| Non-major | 2 (66.7%) |
| Tonic-clonic status epilepticus | 2 (13.3%) |
AF: atrial fibrillation; AFL: atrial flutter; VF: ventricular fibrillation; VT: ventricular tachycardia.
Table 4 compares the seven patients (47%) who survived (survivor group) and were discharged with CPC 1 with the eight patients (53%) who died in-hospital (non-survivor group).
Comparison of the survivor and non-survivor groups.
| Characteristics, n (%) or mean ± SD | Survivor group (n=7) | Non-survivor group (n=8) | p |
|---|---|---|---|
| Gender, male | 5 (71.4%) | 5 (62.5%) | 0.724 |
| Age, years | 38.4±10.1) | 55.1±13.2 | 0.032 |
| CV risk factors | 3 (42.9%) | 7 (87.5%) | 0.077 |
| Hypertension | 2 (28.6%) | 4 (50.0%) | 0.414 |
| Diabetes | 0 (0.0%) | 3 (37.5%) | 0.080 |
| Dyslipidemia | 2 (28.6%) | 3 (37.5%) | 0.724 |
| Active smoking | 2 (28.6%) | 4 (50.0%) | 0.414 |
| BMI ≥25 kg/m2 | 0 (0.0%) | 2 (25.0%) | 0.170 |
| Out-of-hospital cardiac arrest | 5 (71.4%) | 6 (75.0%) | 0.880 |
| VF rhythm at presentation | 6 (85.7%) | 4 (50.0%) | 0.157 |
| CA-CPR, min | 9.4±4.2 | 18.5±15.8) | 0.473 |
| CA-ROSC, min | 22.9±12.9 | 63.8±40.4 | 0.048 |
| CA-TH, min | 348±138 | 336±234 | 0.601 |
| GCS | 5.9±3.3 | 4.5±2.5 | 0.413 |
| Laboratory values | |||
| Hemoglobin, g/dla | 13.8±3.2 | 13.3±1.3 | 0.417 |
| Sodium, mmol/la | 140.7±7.2 | 139.1±3.8 | 0.685 |
| Potassium, mmol/la | 3.5±0.7 | 4.1±1.3 | 0.450 |
| Total bilirubin, μmol/la | 13.3±7.9 | 8.9±5.5 | 0.247 |
| AST, U/la | 145.6±112.3 | 400.8±423.7 | 0.418 |
| ALT, U/la | 83.6±43.2 | 248.3±188.1 | 0.203 |
| NSE, μg/la | 42.6±35.1 | 138.4±133.4 | 0.020 |
| Troponin I, ng/mlb | 16.5±24.8 | 56.8±47.1 | 0.165 |
| BNP, pg/mlb | 339.6±401.2 | 479.7±365.4 | 0.475 |
| Renal function | |||
| Creatinine, mg/dla | 1.1±0.2 | 1.3±0.5 | 0.354 |
| BUN, mmol/la | 6.1±1.4 | 7.7±4.9 | 0.954 |
| eGFR, ml/min/1.73 m2a | 72.7±12.7 | 58.9±22.3 | 0.183 |
| eGFR <60 ml/min/1.73 m2a | 2 (28.6%) | 4 (50.0%) | 0.414 |
| LV function | |||
| Eyeball LV ejection fraction <50% | 0 (0.0%) | 6 (75.0%) | 0.002 |
| Treatment | |||
| PCI | 3 (42.9%) | 6 (75.0%) | 0.221 |
| Implanted cardioverter-defibrillator | 4 (57.1%) | 0 (0.0%) | 0.016 |
| Length of ICU stay, days | 9.0±3.7 | 23.1±32.4 | 0.480 |
| Length of hospital stay, days | 16.1±7.9 | 28.1±35.6 | 0.908 |
BMI: body mass index; BNP: B-type natriuretic peptide; BUN: blood urea nitrogen; CA-CPR: time from collapse to cardiopulmonary resuscitation; CA-ROSC: time from collapse to return of spontaneous circulation; CA-TH: time from collapse to beginning of therapeutic hypothermia; CV: cardiovascular; eGFR: estimated glomerular filtration rate (Modification of Diet in Renal Disease formula); GCS: Glasgow Coma Scale; LV: left ventricular; NSE: neuron-specific enolase; PCI: percutaneous coronary intervention; VF: ventricular fibrillation.
No differences were found between the groups regarding gender (male: 71.4% vs. 62.5%; p=0.724), history of hypertension (28.6% vs. 50.0%; p=0.414), diabetes (0.0% vs. 37.5%; p=0.080); dyslipidemia (28.6% vs. 37.5%; p=0.724), active smoking (28.6% vs. 50.0%; p=0.414), or overweight or obesity (0.0% vs. 25.0%; p=0.170). The location of CA was similar (out-of-hospital: 71.4% vs. 75.0%; p=0.880). Although an initial VF rhythm was more frequent in survivors in this small group of patients, there was no statistical difference between the groups (85.7% vs. 50.0%; p=0.157).
Patients who survived were a mean of 16.7 years younger than those who died (38.4 vs. 55.1 years; p=0.032), had shorter CA-ROSC (22.9 vs. 63.8 min; p=0.048) and lower NSE values (42.6 vs. 138.4 μg/l; p=0.020).
To analyze the clinical predictors for survival, the cut-off values were calculated on the basis of the 50th percentile. Age >48 years was a strong predictor of survival (OR: 18.00; 95% CI: 1.27-255.74; p=0.023). Approaching borderline statistical significance as predictors of mortality were presence of cardiovascular risk factors (OR: 9.33; 95% CI: 0.711-122.57; p=0.077), CA-ROSC >35 min (OR: 4.17; 95% CI: 0.47-36.74; p=0.082) and NSE >76 μg/l (OR: 7.50; 95% CI: 0.76-74.16; p=0.082) (Table 5).
Cut-off values of clinical predictors of mortality.
| OR | 95% CI | p | |
|---|---|---|---|
| Age >48 years | 18.00 | 1.27-255.74 | 0.023 |
| ≥1 CV risk factors | 9.33 | 0.711-122.57 | 0.077 |
| CA-ROSC >35 min | 4.17 | 0.47-36.74 | 0.082 |
| NSE >76 μg/l | 7.50 | 0.76-74.16 | 0.082 |
CA-ROSC: time from collapse to return of spontaneous circulation; CI: confidence interval; CV: cardiovascular; NSE: neuron-specific enolase; OR: odds ratio.
Within the last decade outcomes after CA have improved, thanks to a combination of various measures. TH has shown benefit, however specific predictors of outcome in hypothermia-treated CA patients are missing.
In our study we used a target temperature of 33°C in all patients, as further evidence has supported the findings of Holzer et al. and Bernard et al.5,6 However, a recent international randomized controlled trial by Nielsen et al.9 completely contradicts the findings of all previous studies. They reported no significant difference in outcome between out-of-hospital CA patients treated with hypothermia at target temperatures of 33°C or 36°C. New guidelines have established targeted temperature management at a constant temperature between 32°C and 36°C for at least 24 hours.11 They state that the terms “target temperature management” or “temperature control” are now preferred over the previous term “therapeutic hypothermia” and that a priority in temperature control should be to avoid hyperthermia. Nevertheless, it is clear that weak recommendations and low-quality evidence in some points suggest that doubts remain and that further large high-quality trials are needed.
Post-cardiac arrest syndrome in patients who remain comatose after CA is a complex set of pathophysiological processes consisting of systemic ischemia-reperfusion injury, brain injury, and myocardial depression, as well as ongoing injury caused by the precipitating etiology of the arrest.17 In this field TH has been the cornerstone in neurological improvement. In this study we investigated mortality, neurological outcome and possible predictors of poor outcome. Our overall survival to discharge was 47%, with all patients surviving to discharge having a good neurological outcome, defined as a CPC of 1. In the first two major clinical trials that provided direct evidence of a benefit of TH, 49% and 55%, respectively, who were treated with hypothermia survived and had a favorable neurological recovery at hospital discharge.5,6
Assessment of neurological function and prognosis is challenging in patients undergoing TH and some authors suggest a multimodal approach based on neurological examination, continuous electroencephalographic monitoring, somatosensory-evoked potential, magnetic resonance and CT imaging and serum markers of neuronal damage.18
An early CT scan is commonly used to rule out an unexpected intracerebral cause of coma in patients who remain comatose after cardiac arrest, and CT after TH gives important information about neurological outcome and longtime survival.18 In the current series CT was not routinely performed at admission, or after TH as a prognostic tool. In those who underwent CT after TH, cerebral edema and hypoxic encephalopathy were associated with a poor outcome. Current resuscitation guidelines recognize echocardiography as a valuable diagnostic tool in the early identification of the precipitating cause of CA,11,19 which is why it was performed in all except one of our patients in the after-arrest setting. However the exam was a point-of-care focused study, with non-standard echocardiographic views, in patients under mechanical ventilation and inotropic support. This limited approach could have led to important findings being missed or misinterpreted, particularly with left ventricular ejection fraction being overestimated due to inotropic support leading to myocardial hypercontractility.
Various interesting biomarkers for prognosis after CA have been studied, including S100B protein,20 glial fibrillary acidic protein, neurofilaments, and organ-specific micro-RNA.21 The most extensively studied biomarker is NSE. A large Dutch study, prior to the era of TH, identified an NSE value >33 μg/l within 72 hours of CA as a reliable marker for poor outcome.22 While some recent studies have questioned the value of NSE for prognostication in hypothermia-treated patients and consider it insufficiently predictive,23 other studies support its use.24,25 Cut-off points for predicting a poor outcome with no false positives vary greatly (from 9 to 97 μg/l).26 Studies testing NSE as a predictor of poor outcome in comatose patients after CA treated with TH have reported cut-off values at 48 hours of 28 ng/ml and 33 ng/ml.24,25 In our population, NSE levels were 95.8 μg/l higher in those who died (42.6±35.1 vs. 138.4±133.4 μg/l, p=0.020). An NSE cut-off of 76 μg/l appears to separate the two groups at close to statistical significance. This cut-off, higher than in previous studies, could be explained by differences in assay procedures and time of blood sampling (in our study within 72 hours). If only NSE values within the first 48 hours are considered, the mean is 44.2 μg/l, approaching those in previous studies. When and how to prognosticate, in particular which biomarkers should be used, is a question that requires more data. Furthermore, any specific absolute cut-off value proposed to provide neuroprognostication should only be used in conjunction with other prognostic variables.
N-terminal pro-B-type natriuretic peptide (NT-proBNP) and troponin I have been shown to be important biomarker predictors for death in CA patients.27 In our study higher B-type natriuretic peptide (BNP) (339.6±401.2 vs. 479.7±365.4 pg/ml, p=NS) and peak troponin I (16.5±24.8 vs. 56.8±47.1 ng/ml, p=NS) were found in the non-survivor group, but without statistical significance, due to the small number of patients enrolled.
In our results gender, GCS, and location and initial rhythm of CA were not predictors of mortality. While some studies show female gender as an independent predictor for both less use of TH and poor outcome in patients with a shockable rhythm,28 others identify female gender as a positive predictor for survival to hospital arrival after an out-of-hospital CA, despite worse survival to hospital discharge.29 The abnormal distribution of GCS scores in our study, with a median of 3, meant it had no influence on mortality, in contrast to other studies in which one of the best predictors was low GCS at admission.30 Although in-hospital CA has a weaker recommendation to use TH,10,11 in our study, as well as in another Portuguese study,31 there was no difference in survival between in-hospital and out-of-hospital CA. One of the reasons for this could be less effective prehospital care affecting in-hospital mortality.
A Scandinavian registry enrolling 986 patients reported 61% survival in the witnessed ventricular tachycardia/VF arrest group and 56% good neurological outcome.30 Consistent with these findings, we report 66.7% (n=10) of patients with shockable initial rhythm and overall survival with a good neurological outcome in 60% (n=4) of these. These data highlight the expected survival rate and hence the superior impact of TH for these patients compared with those with a non-shockable CA rhythm. There are multiple causes of CA due to asystole and pulseless electrical activity, and the underlying medical problems are more difficult to treat. In this context, some authors have found that hypothermia was not of benefit in terms of survival or good neurological outcome if the CA was related to a non-shockable rhythm.32 Others have concluded that a positive impact on survival is likely.33 It is still unclear whether TH benefits non-shockable victims if the pathophysiology of cerebral metabolism and dysfunction is different or if a different target temperature is needed in such patients.
Patients who survived in our study were a mean of 16.7 years younger than those who died and patients aged over 48 years had a markedly poorer outcome. Older age remains a negative predictor for TH use28 as well as predicting worse survival.28,30 Advanced age is a common reason for withholding TH in unconscious out-of-hospital CA patients.34
Emergency coronary angiography giving an opportunity for emergency percutaneous coronary intervention (PCI) is an established and widely implemented first-line treatment in patients with ST-segment elevation myocardial infarction (STEMI) complicated with CA.12 However, it remains unclear whether an early invasive strategy is beneficial in all comatose survivors of CA. Selection of patients with a high likelihood of coronary artery disease may be challenging. In our study, all but one patients (n=14; 93.3%) received emergency coronary angiography and 60% (n=9) subsequently received PCI. Despite this, we consider that the benefits (marginal in many cases) and potential procedure-related complications of emergency coronary angiography and emergency PCI in comatose patients should be balanced against those of a less invasive strategy.
Although in our population coronary angiography and PCI were performed before TH, most centers combine early PCI with simultaneous initiation of TH, which has also been shown to be feasible without increasing door-to-balloon time. It seems reasonable that hypothermia should be initiated as early as possible, however there is a study showing that later initiation of cooling is still effective in mitigating neuronal damage.30 The mean CA-TH in this study (90 min) was considerably shorter than in ours (342 min), showing how much our performance could be improved. In fact, the technique of TH requires a learning period and specific skills. Although time is crucial at every step of the process, in our study there was no difference in CA-TH between survivor and non-survivor groups (348±138 vs. 336±234 min, p=NS). Our findings are similar to those of the Hypothermia After Cardiac Arrest (HACA) study, in which the median interval between ROSC and target temperature was eight hours (480 min),5 due to rapid induction of TH.
Comatose CA patients are admitted to the ICU with a broad range of baseline probability of survival, mainly due to differences in resuscitation times. The time from collapse to ROSC is an important predictor of survival, as confirmed by our results, in which this was a mean of 40.9 min longer in those who died and a cut-off of 35 min approached statistical significance to differentiate the two groups. The cut-offs are not very different in other studies, in which the outcome for patients with time to ROSC of over 30 min (after calling for an ambulance) is dismal35 and time from collapse to ROSC of over 25 min is strongly associated with poor outcome.36 Although longer time to ROSC was a predictor of poor outcome, on the basis of absolute numbers it could not be used as a sole predictor of outcome, and other factors such as the quality of CPR may be equally important.
LimitationsCertain limitations to our retrospective, registry-based study should be recognized.
First of all, the small sample size may limit the analysis, which is clearly underpowered to assess major differences. However this is the largest series so far published in the Portuguese population after the implementation of TH and the patient characteristics are similar to previous reports.
The overall low use of objective prognostic tools in our patient group was in agreement with common practice in the ICU at the time of the study.
Finally, we studied patients from a single center, and therefore the generalizability of our results to other centers may be questioned.
ConclusionsThe high morbidity and mortality associated with CA means the recommendations in this article should be viewed in light of the very poor prognosis in CA patients. Due to the complexity and heterogeneity of these patients, performing well-controlled randomized trials is a challenge, and thus there are very few proven interventions. Our study results support the findings of prior studies conducted on TH-treated CA patients: 53% of all patients died and 47% of all patients were discharged with a favorable outcome. Predictors for death were higher age, previous cardiovascular risk factors, longer CA-ROSC and higher NSE levels at admission; a relation between shockable rhythm or out-of-hospital CA and mortality was not fully documented.
Several questions remain unanswered: the optimal time to begin TH; the target temperature and duration of TH; the best local cooling practices; the effect of rewarming rate; and the possibility that some subgroups of patients may benefit from a specific target temperature.
No single test can predict a poor prognosis with absolute certainty and it will be difficult to establish cut-off values supporting the decision whether to induce hypothermia. Furthermore, the use of TH may complicate assessment of the neurological outcome. The optimal approach to prognosticate CA patients treated with TH has not been fully resolved. This is an issue of central importance, since it can be ethically justifiable to limit care or withdraw support only when a poor prognosis is reasonably certain.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right of privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.