Acute kidney injury (AKI) is a pathological phenomenon with a negative impact on outcomes in different clinical scenarios. Its mechanism in acute coronary syndrome (ACS) is not completely understood, and measures to prevent it are not uniform. We set out to study the incidence, clinical relevance, predictors and possible implications for patient management of AKI in ACS.
MethodsUsing data from a multicenter national registry on ACS, we retrospectively analyzed predictors of AKI and its impact on outcomes (in-hospital complications and one-year mortality). All ACS types were included. AKI was defined as an increase in serum creatinine of ≥0.3 mg/dl (≥26.4 μmol/l) and/or by ≥1.5 times baseline.
ResultsA total of 7808 ACS patients were included in the analysis, 1369 (17.5%) of whom developed AKI. AKI was shown to be an independent predictor of in-hospital major bleeding (odds ratio [OR] 2.09; 95% confidence interval [CI] 1.19-3.64; p=0.01), mortality (OR 4.72; 95% CI 2.94-7.56; p<0.001) and one-year mortality (hazard ratio 2.01; 95% CI 1.51-2.68; p<0.001). The incidence of AKI was associated with older age, history of hypertension, renal failure and stroke/transient ischemic attack, Killip class >1 on admission and left ventricular ejection fraction <50%. Performance of coronary angiography or angioplasty were not associated with AKI. Diuretics during admission were predictors of AKI only in patients in Killip class 1.
ConclusionsAKI is an important finding in ACS, with a significant impact on hard clinical endpoints such as in-hospital and one-year mortality. It is associated with easily identifiable clinical factors and an invasive strategy does not increase its incidence.
A lesão renal aguda (LRA) é um fenómeno patológico que acontece em várias situações clínicas. A sua fisiopatologia no contexto de síndrome coronária aguda (SCA) não é completamente conhecida, pelo que as medidas de prevenção não são uniformes. Neste trabalho, estudamos a LRA em relação ao significado clínico, preditores e possíveis implicações para o tratamento no contexto de SCA.
MétodosUtilizando dados de um registo nacional multicêntrico de SCA, analisámos, retrospetivamente, os preditores de LRA e o impacto na clínica através de complicações intra-hospitalares (IH) e mortalidade a um ano. A LRA foi definida como aumento de creatinina sérica de ≥0,3 mg/dl e/ou 1,5 vezes o seu valor basal durante o internamento.
ResultadosForam incluídos 7808 doentes com SCA na análise, 1369 (17,5%) dos quais desenvolveram LRA. A LRA revelou ser preditor independente de hemorragia major IH (odds ratio [OR] 2,09; intervalo de confiança 95% [IC] 1,19-3,64; p=0,01), mortalidade IH (OR 4,72; IC 2,94-7,56; p<0,001) e mortalidade a um ano (hazards ratio 2,01; IC 1,51-2,68; p<0,001). A incidência de LRA associa-se ao aumento da idade, hipertensão arterial, insuficiência renal e AVC/AIT, Killip-Kimball (KK) >1 na admissão e fração de ejeção ventricular esquerda <50%. A realização de coronariografia ou angioplastia não se associaram a um aumento de LRA. A utilização de diuréticos foi preditora de LRA apenas em doentes KK 1.
ConclusõesA LRA é um achado importante no contexto de SCA, com impacto clínico significativo, nomeadamente mortalidade intra-hospitalar e a um ano. A LRA associa-se a características clínicas facilmente identificáveis e a estratégia invasiva não aumenta a sua incidência.
Acute kidney injury (AKI) is a pathological phenomenon that has changed in name and definition in recent years. The initial concepts of acute kidney failure and renal impairment were difficult to compare between studies, so there was a need to standardize a definition of what can be described as an “acute decline of kidney function as a consequence of a pathological insult”.
In 2004, the Acute Dialysis Quality Initiative group proposed the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) classification for acute renal failure,1 which included serum creatinine (SCr) and/or urine output criteria to define three levels of renal dysfunction and two clinical outcomes.
In 2007, the Acute Kidney Injury Network group proposed the term ‘acute kidney injury’ and a new definition for the entire spectrum of acute renal failure, based on the previous RIFLE criteria.2 It included three stages of AKI, also taking into account SCr and/or urine output.
The latest definition was published in 2012 by the Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group.3 It combines the previous definitions: AKI is present if SCr increases by ≥0.3 mg/dl (≥26.5 μmol/l) in 48 hours or by ≥1.5 times baseline within seven days, or if urine volume is <0.5 ml/kg per hour for six hours.
These criteria were largely derived from critically ill patients in intensive care units, with AKI of varying etiologies, and KDIGO appears to be a better predictor of in-hospital mortality than RIFLE.4 Understanding of AKI in acute coronary syndrome (ACS) is improving, but is still limited. This study aimed to determine the incidence of AKI in ACS patients, to assess its impact on clinical outcomes, and to determine its predictors.
MethodsStudy population and data managementThis was a retrospective study derived from the Portuguese Registry on Acute Coronary Syndromes (ProACS). Data are collected at a national level from all participating centers and entered into a dedicated web-based platform by the attending physician or other designated person at the time of discharge and at one-year follow-up. Information is recorded on demographics, clinical profile, clinical presentation, laboratory and imaging results, interventions, medication and in-hospital complications (reinfarction, congestive heart failure, shock, atrial fibrillation, mechanical complication, atrioventricular block, sustained ventricular tachycardia, cardiac arrest, stroke, major bleeding and death). Patients with all types of ACS, namely unstable angina, non-ST elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI), are included. Patients on dialysis are excluded. The period from October 1, 2010 to March 23, 2015 was analyzed, in which a total of 11812 patients were included.
AKI was defined as an increase in SCr of ≥0.3 mg/dl (≥26.4 μmol/l) and/or by ≥1.5 times baseline during the admission period. Chronic renal failure was defined as serum creatinine >2.0 mg/dl prior to admission or a history of renal transplant. Major bleeding included severe or life-threatening bleeding, as defined by the GUSTO criteria.5
Statistical analysisBaseline characteristics were compared between patient groups (with and without AKI), using Student's t tests or Mann-Whitney U tests for continuous variables and the chi-square test or Fisher's exact test for categorical variables, as appropriate. Continuous variables were expressed as mean ± standard deviation (SD) or median (Q1-Q3), and dichotomous variables as percentages.
A multivariate logistic regression analysis was performed to assess independent predictors of AKI and the influence of AKI on in-hospital mortality and complications. A Cox proportional hazards model was used to assess the influence of AKI on cumulative mortality at one year. Odds ratios (OR) and hazard ratios (HR) were calculated with 95% confidence intervals.
IBM SPSS Statistics version 19 was used for the statistical analysis and a p value of <0.05 was considered statistically significant.
ResultsBaseline characteristicsThe baseline characteristics of the study population are summarized in Table 1, which also shows univariate associations with development of AKI. A total of 7808 patients were included in the analysis, after confirmation of availability of complete patient data required for this study. About three-quarters of the patients were male. AKI was associated with older age and higher prevalence of hypertension and diabetes. Interestingly, AKI patients were less frequently smokers and less frequently underwent coronary angiography and percutaneous coronary intervention (PCI). To assess the true influence of these clinical and procedural variables on development of AKI, a multivariate logistic regression analysis was performed.
Baseline and admission characteristics of the study population.
| Variable | Total (n=7808) | No AKI (n=6439) | AKI (n=1369) | p |
|---|---|---|---|---|
| Mean age (years) | 66±13 | 65±13 | 73±12 | <0.001 |
| Male (%) | 72.1 | 73.3 | 66.5 | <0.001 |
| BMI (kg/m2) | 27.4±4.3 | 27.4±4.3 | 27.4±4.5 | 0.954 |
| Patient delay (min)a | 157 [80; 340] | 150 [78; 321] | 194.5 [93.5; 440.5] | <0.001 |
| Smoking (%) | 27.4 | 30.0 | 15.0 | <0.001 |
| Hypertension (%) | 69.7 | 67.5 | 80.0 | <0.001 |
| Diabetes (%) | 30.3 | 28.8 | 37.5 | <0.001 |
| Dyslipidemia (%) | 58.5 | 58.5 | 58.2 | 0.856 |
| Previous MI (%) | 20.5 | 19.9 | 23.6 | 0.002 |
| Previous PCI (%) | 14.4 | 14.5 | 13.8 | 0.529 |
| Previous CABG (%) | 4.9 | 4.9 | 4.7 | 0.691 |
| Previous renal failure (%) | 4.8 | 3.2 | 12.8 | <0.001 |
| Previous PAD (%) | 5.5 | 4.8 | 8.7 | <0.001 |
| ACS type | ||||
| STEMI (%) | 41.2 | 41.1 | 41.7 | 0.674 |
| – anterior (%) | 48.5 | 46.6 | 57.3 | <0.001 |
| – inferior (%) | 50.4 | 52.4 | 41.0 | <0.001 |
| New LBBB (%) | 1.2 | 1.0 | 1.8 | 0.137 |
| NSTEMI (%) | 48.9 | 49.2 | 47.6 | 0.282 |
| UA (%) | 6.0 | 6.5 | 3.2 | <0.001 |
| Physical examination on presentation | ||||
| HR (bpm) | 78±20 | 77±19 | 82±23 | <0.001 |
| Systolic BP (mmHg) | 139±29 | 139±29 | 139±31 | 0.794 |
| Diastolic BP (mmHg) | 79±17 | 79±17 | 78±18 | 0.015 |
| Killip class II-IV | 15.2 | 11.0 | 34.8 | <0.001 |
| BNP (pg/ml) | 170.5 [67; 643] | 138 [54; 334] | 529 [211; 1140] | <0.001 |
| Coronary angiography (%) | 87.3 | 88.7 | 80.3 | <0.001 |
| Extent of CAD (%)b | ||||
| 0 vessels | 6.6 | 7.0 | 4.4 | 0.002 |
| 1-vessel | 42.8 | 44.3 | 34.9 | <0.001 |
| 2-vessel | 28.8 | 28.2 | 32.2 | 0.009 |
| 3-vessel | 21.8 | 20.5 | 28.5 | <0.001 |
| PCI performed (%) | 65.4 | 66.9 | 58.2 | <0.001 |
| LVEF <50% (%) | 36.4 | 32.5 | 55.0 | <0.001 |
| In-hospital medication | ||||
| ACEIs/ARBs (%) | 87.1 | 87.9 | 83.2 | <0.001 |
| Diuretics (%) | 30.5 | 23.8 | 62.0 | <0.001 |
| Statins (%) | 95.5 | 95.9 | 93.7 | <0.001 |
| In-hospital complications | ||||
| Reinfarction (%) | 1.0 | 1.1 | 1.0 | 0.737 |
| Congestive heart failure (%) | 10.6 | 10.8 | 9.2 | 0.085 |
| Atrial fibrillation (%) | 3.5 | 3.4 | 4.0 | 0.266 |
| Stroke (%) | 0.7 | 0.6 | 0.7 | 0.695 |
| Major bleeding (%) | 1.3 | 1.0 | 2.4 | <0.001 |
| AV block (%) | 2.6 | 2.6 | 2.5 | 0.877 |
| Sustained VT (%) | 1.3 | 1.2 | 1.8 | 0.053 |
ACEIs: angiotensin-converting enzyme inhibitors; ACS: acute coronary syndrome; AKI: acute kidney injury; ARBs: angiotensin receptor blockers; AV: atrioventricular; BMI: body mass index; BNP: brain-type natriuretic peptide, expressed in pg/ml as median [1st quartile; 3rd quartile]; BP: blood pressure; CABG: coronary artery bypass grafting; CAD: coronary artery disease; HR: heart rate; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; OR: odds ratio, expressed as OR (95% confidence interval); PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; VT: ventricular tachycardia.
Defined as time from pain onset to first medical contact, and expressed in min as median [1st quartile; 3rd quartile].
Defined as number of main coronary arteries (left main [LM], left anterior descending [LAD], left circumflex [LCX], right coronary [RCA]) with ≥50% stenosis: 1-vessel=LAD, LCX or CD; 2-vessel: LM, LM+LAD, LM+LCX, LM+LAD+LCX, DA+CX, LAD+RCA or LCX+RCA; 3-vessel: LAD+LCX+RCA, LM+RCA, LM+LAD+RCA, LM+LCX+RCA or LM+LAD+LCX+RCA.
The following characteristics were included in the logistic regression analysis: demographics, ACS type, risk factors and comorbidities, Killip class on admission, performance of angiography, angiography results, performance of percutaneous coronary intervention (PCI), left ventricular ejection fraction (LVEF), and previous medication and medication during admission (angiotensin converting enzyme inhibitors [ACEIs], angiotensin receptor blockers [ARBs] and diuretics). Results are summarized in Table 2. Older age and history of hypertension, renal failure and stroke/transient ischemic attack were identified as independent predictors of AKI, as was Killip class >1 on admission and LVEF <50%. The performance of angiography and/or PCI did not increase the incidence of AKI. Statins and ACEIs or ARBs did not show a statistically significant independent effect on the development of AKI, although a tendency towards a protective effect was noted for both drug types. Diuretics during the admission period were shown to be independent predictors of AKI. However, a strong interaction between diuretics and Killip class was noted, i.e. diuretics were independent predictors of AKI if Killip class on admission was I (OR 2.92 [2.46-3.47]) but not if it was II-IV (OR 1.32 [0.93-1.88]).
Multivariate logistic regression assessing predictors of development of acute kidney injury.
| Predictor | Beta | SE | p | OR (95% CI) |
|---|---|---|---|---|
| Age | 0.029 | 0.003 | <0.001 | 1.03 (1.02-1.04) |
| Previous renal failure | 0.834 | 0.131 | <0.001 | 2.30 (1.78-2.98) |
| Hypertension | 0.275 | 0.089 | 0.002 | 1.32 (1.11-1.57) |
| Previous stroke/TIA | 0.238 | 0.112 | 0.034 | 1.27 (1.02-1.58) |
| Killip class on presentation >1 | ||||
| Diuretics during admission | 0.361 | 0.097 | <0.001 | 1.43 (1.19-1.74) |
| No diuretics during admission | 1.154 | 0.172 | <0.001 | 3.17 (2.26-4.45) |
| Drugs during admission period: | ||||
| Diuretics | ||||
| Killip class 1 | 1.072 | 0.088 | <0.001 | 2.92 (2.46-3.47) |
| Killip class >1 | 0.279 | 0.179 | 0.119 | 1.32 (0.93-1.88) |
| ACEIs/ARBs | -0.119 | 0.104 | 0.251 | 0.89 (0.72-1.09) |
| Statins | -0.180 | 0.156 | 0.248 | 0.83 (0.61-1.13) |
| Angiography | -0.501 | 0.189 | 0.008 | 0.61 (0.42-0.88) |
| PCI | -0.037 | 0.095 | 0.698 | 0.96 (0.80-1.16) |
| LVEF <50% | 0.329 | 0.076 | <0.001 | 1.39 (1.20-1.61) |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; CI: confidence interval; LVEF: left ventricular ejection fraction; OR: odds ratio; PCI: percutaneous coronary intervention; SE: standard error; TIA: transient ischemic attack.
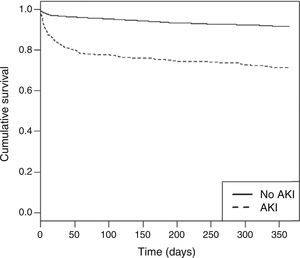
AKI was associated with increases in in-hospital major bleeding, shock and mechanical complications. Of all in-hospital complications included in the study, after multivariate regression analysis only major bleeding was independently predicted by AKI, with OR 2.09 (95% CI 1.19-3.64) (p=0.01). AKI was also an independent predictor of mortality, both in-hospital (Table 3), and at one-year follow-up (Figure 1, Supplementary Table 1) (HR 2.01; 95% CI 1.51-2.68; p<0.001). The type of ACS does not seem to significantly affect the impact of AKI on these outcomes. LVEF is still an excellent tool for risk stratification, for both in-hospital and one-year mortality.
Multivariate logistic regression assessing predictors of in-hospital mortality.
| Predictor | Beta | SE | p | OR (95% CI) |
|---|---|---|---|---|
| AKI | 1.551 | 0.241 | <0.001 | 4.72 (2.94-7.56) |
| Female | 0.598 | 0.238 | 0.012 | 1.82 (1.14-2.90) |
| Age | 0.046 | 0.011 | <0.001 | 1.05 (1.02-1.07) |
| STEMI | 1.060 | 0.256 | <0.001 | 2.89 (1.75-4.76) |
| Valve disease | 1.654 | 0.379 | <0.001 | 5.23 (2.49-10.98) |
| PAD | 0.988 | 0.382 | 0.010 | 2.68 (1.27-5.68) |
| Killip class on presentation | ||||
| II | 0.481 | 0.282 | 0.088 | 1.62 (0.93-2.81) |
| III | 0.734 | 0.388 | 0.058 | 2.08 (0.97-4.45) |
| IV | 2.228 | 0.444 | <0.001 | 9.28 (3.88-22.17) |
| Multivessel disease | 0.612 | 0.249 | 0.014 | 1.84 (1.13-3.00) |
| LVEF | ||||
| Mildly depressed | 0.176 | 0.371 | 0.635 | 1.19 (0.58-2.47) |
| Moderately depressed | 1.344 | 0.309 | <0.001 | 3.84 (2.09-7.03) |
| Severely depressed | 2.378 | 0.328 | <0.001 | 10.78 (5.67-20.50) |
AKI: acute kidney injury; CI: confidence interval; LVEF: left ventricular ejection fraction; OR: odds ratio; PAD: peripheral arterial disease; SE: standard error; STEMI: ST-elevation myocardial infarction.
The major findings of this study are that AKI is an independent predictor of major bleeding and mortality (both in-hospital and at one year) and that it is independently associated with easily identifiable clinical variables, such as Killip class >1 at admission, previous renal dysfunction, older age and depressed LVEF. AKI patients tend to be older and more often hypertensive and diabetic, conditions known to affect renal functional reserve, and also tend to have more complex coronary artery disease.
STEMI patients seem to be at greatest risk for developing AKI. This finding is to be expected, since they represent patients with ACS of greatest hemodynamic impact and/or with lowest hemodynamic and renal functional reserve. Interestingly, diuretic use was also an independent predictor of AKI, but only in Killip class 1 patients, not in Killip class >1, which may be a reflection of its benefit only in the presence of marked fluid overload. Statins did not seem to protect against AKI, although these drugs at high doses have been shown to have an important role in preventing AKI.6–8 ACEIs/ARBs also failed to demonstrate a significant protective effect.
Also of note, the performance of angiography and angioplasty were not associated with increased development of AKI, despite the associated use of nephrotoxic contrast agents. In this context, it could be postulated that the hemodynamic benefit of revascularization may outweigh the deleterious effect of contrast use. Increasingly used prophylactic measures for AKI, such as vigorous hydration and high-dose statins, may also have played a part in these results.
Our findings are generally in line with those of previously published studies in several respects. The negative impact of AKI on short- and long-term outcomes of patients undergoing PCI has been demonstrated by several groups, particularly in terms of in-hospital and long-term mortality.9–14 Similarly, in ACS patients development of AKI and its severity are associated with a progressively higher risk of in-hospital morbidity and mortality.15 The commonly used scores for AKI diagnosis and staging have also been tested in ACS, and the KDIGO criteria appear to be better than RIFLE for predicting early and late mortality in myocardial infarction (MI).16
In the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) registry,17 in which most patients had ACS (72%), both cardiovascular and renal events also increased with the presence and severity of AKI. AKI in the setting of ACS is frequently interpreted as contrast-induced, and in this context it has been shown to be a predictor of mortality in-hospital and in follow-up (median 94 months).13
The importance of contrast for the development of AKI in ACS is still the subject of debate. Other mechanisms, with both prerenal and renal pathophysiology, have been proposed, such as the hemodynamic impact of the ACS, bleeding complications, atheroembolic phenomena, drug toxicity and acute hyperglycemia.18 BNP levels have been shown to be associated with the development of AKI, possibly reflecting both cardiac and renal impairment in the context of ACS.19 Radial access has recently been shown in a meta-analysis20 to be significantly less associated with AKI than femoral access, possibly related to reduced bleeding complications. The AKI-MATRIX sub-study21 will probably add important information in this regard. Several groups have recently developed scores to predict the development of AKI after PCI.22–24 Interestingly, they are very robust, despite being based on preprocedural data only, and in all of them AKI appears to be more frequent in ACS, including MI, than in stable patients. This suggests there are important mechanisms of AKI in ACS patients, other than contrast use or volume. In this regard, a recent study by McDonald et al. suggests that iodinated contrast use is not associated with development of AKI in patients undergoing CT.25
Although it is probably not the main mechanism, there is still evidence of the deleterious effect of contrast material on renal function in ACS.26 An early invasive strategy in NSTEMI has been reported to be associated with a small increase in AKI, but not dialysis or progression to end-stage kidney disease, and it still reduced long-term mortality.27 In primary PCI, contrast volume is still an important determinant of AKI,28 regardless of preprocedural risk,29 which supports the idea of keeping contrast use to a reasonable minimum.30
Study limitationsThe main limitations of this study derive from its retrospective design, which limits available data. Data on preventive measures for AKI were not available, nor on the type and volume of contrast used. However, pooling all invasively treated patients together did not show an increased incidence of AKI, regardless of the contrast volume used, which is assumed to have been kept to a minimum as standard practice. Some AKI cases may have been missed, because SCr on admission may not reflect true baseline levels, i.e. AKI might already be ongoing, since time from symptom onset to admission was variable. It would also be interesting to study AKI with other biomarkers, like NGAL,31,32 cystatin C33 and KIM-1,34 which are potentially more accurate indicators of AKI than creatinine. Information on statin doses was not available, which may be responsible for the absence of a protective effect of statins in our results.
ConclusionsAKI is an important finding in the ACS context, with impact on hard clinical endpoints, such as major bleeding and mortality. Easily identifiable markers of vulnerable patients are Killip class >1 at admission, previous renal dysfunction, older age and depressed LVEF. Care should be taken when using diuretics, which may be deleterious in the absence of fluid overload. Mechanisms other than contrast toxicity are likely to have an important role. Invasive management of ACS does not seem to increase AKI. However, keeping contrast volume to a reasonable minimum is still advisable.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank all contributors from all participating centers in ProACS, as well as the Portuguese Society of Cardiology for this ever-growing initiative.