Post-exercise hypotension (PEH) is a phenomenon characterized by a decrease in systolic and/or diastolic blood pressure after a single session of physical exercise,1 in comparison both to resting values (pre-exercise) and to a control condition (without exercise), with no clinical symptoms (such as syncope).
The clinical relevance of PEH is well established, since the reductions in blood pressure appear to be large and to last for several hours,2–4 thus representing an acute reduction in cardiovascular risk. Another aspect that has gained prominence is the probable association between acute and chronic blood pressure responses to exercise.5 In other words, it has been observed that individuals who experience a greater acute blood pressure reduction also experience greater reductions after a training program.6 As a result, PEH after different types of exercise has been widely analyzed.
The guidelines for blood pressure control generally recommend continuous aerobic exercise,7,8 since a large body of evidence has demonstrated its hypotensive effects in normotensive, prehypertensive and hypertensive patients.2,3,8 However, high-intensity interval exercise (HIIE) has more recently attracted the attention of researchers and exercise professionals, mainly due to its positive effects in improving maximum oxygen consumption at similar or higher levels compared to those observed after a continuous exercise (CE) program.9
Regarding the acute effects of HIIE on blood pressure, a recent systematic review and meta-analysis demonstrated that an HIIE session resulted in a greater magnitude of PEH for both systolic and diastolic blood pressure than a CE session.10 This finding is interesting from a clinical and practical standpoint, since it suggests that HIIE may have an important role in blood pressure control and can therefore be viewed as an alternative or complement to the guidelines’ current recommendations.
Despite the clinical relevance of PEH, the physiological mechanisms which may explain this phenomenon are not fully understood. Blood pressure can be expressed as cardiac output (CO) (the product of heart rate [HR] and stroke volume [SV]) times systemic vascular resistance (SVR); hence, reductions in blood pressure can be explained by both central (reductions in CO) and peripheral changes (reductions in SVR). A review by Brito et al.2 identified studies which observed PEH due to reductions in both CO and SVR, concluding that the characteristics of the exercise protocol may be one factor that can explain such heterogeneity. Most of the studies included in this review used CE, and there is still little discussion of the possible mechanisms underlying the acute reduction in blood pressure after an HIIE session.
Thus, the purpose of this editorial comment is to describe the potential physiological mechanisms underlying hypotension following HIIE.
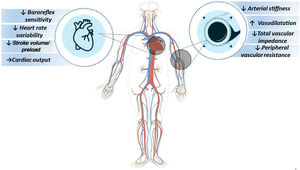
Mechanisms related to post-exercise hypotension after high-intensity interval exerciseStudies have shown that one of the mechanisms involved in the hypotensive response after HIIE is modulation of cardiac function. Increased baroreflex sensitivity at basal levels of neurohumoral regulators in the heart can increase the frequency of firing in the nucleus of the solitary tract by exciting neurons of the nucleus ambiguus and the dorsal nucleus of the vagus nerve, which at the same time inhibit the sympathetic neurons of the rostral ventrolateral medulla, respectively culminating in an increase in vagal tone (parasympathetic nervous system) and a decrease in sympathetic tone, in turn resulting in a reduction in heart rate variability and a beta-antagonistic effect in the heart.11 Thus, as an underlying mechanism of heart rate reduction, the hemodynamic pattern ends by being modified by the reduction in stroke volume and/or a reduction in or maintenance of cardiac output.
Another mechanism involved is vascular function; as blood pressure is based on the product of cardiac output and systemic vascular resistance, and reduction of the latter is related to afterload, along with stroke volume, the regulation of peripheral vascular function will have a direct influence on this response (Figure 1). Systemic vascular resistance can be controlled by alpha 1-adrenergic receptor innervation, local relaxing (via nitric oxide and prostacyclin) or contractile factors (endothelin and angiotensin II), circulating factors such as catecholamines and angiotensin II, and metabolites such as O2, H+ and adenosine.12 Thus, an increase in vasodilation, along with reductions in peripheral vascular stiffness, total vascular impedance, arterial stiffness, and peripheral sympathetic tone as post-exercise responses, will result in a significant blood pressure reduction.13–16
Research has sought to identify the effects of HIIE in the interactions between cardiac, vascular, neuroendocrine, and/or renal mechanisms in different clinical populations.13–18 Analysis of the mechanisms potentially associated with PEH in normotensive adults found that there was a larger reduction in peripheral vascular resistance, stroke volume and cutaneous vascular resistance, and greater blood flow to the skin, after an HIIE session than after CE.13,15 Lacombe et al.17 demonstrated that a larger reduction in baroreflex sensitivity and heart rate variability was observed in prehypertensive men after an HIIE session than following CE.
Moreover, Morales-Palomo et al.13 reported larger reductions in stroke volume, systemic vascular resistance and cutaneous vascular resistance, and greater blood flow to the skin, in hypertensive patients after an HIIE session compared to CE. More recently, Costa et al.14 observed that hypertensive older women showed a reduction in peripheral vascular resistance 60 minutes after an HIIE session compared to the control session. This response was not seen after a CE session. The effect of HIIE on the reduction of systemic vascular resistance is mainly due to secretion of vasodilator substances, and by reduction of peripheral sympathetic activity.
Currie et al.16 demonstrated a significant improvement in endothelial function in patients with coronary artery disease after 60 minutes of HIIE. A similar result was found in a study by Lima et al.,18 in which an HIIE session led to a significant increase in the diameter of the brachial artery of patients with heart failure at 30 minutes of post-exercise recovery.
As can be seen, few studies have analyzed the central or peripheral mechanisms associated with hypotension following HIIE; furthermore, there is no evidence on the exact mechanisms involved in PEH. Although these results have important clinical implications for understanding PEH in different clinical groups in response to HIIE, they need to be analyzed carefully, as they cannot necessarily be extrapolated to prehypertensive or hypertensive populations.
It is important to highlight that a reduction of 2 mmHg in resting blood pressure represents a 6% reduction in mortality from stroke, 4% in coronary artery disease, and 3% in total mortality.19 Thus, since aerobic exercise results in PEH of magnitudes greater than 2 mmHg, it can be said that there is an acute reduction in cardiovascular risk after performing this type of exercise.
Despite the significant degree of PEH in response to HIIE, it is worth noting that its clinical relevance is also dependent on how long it lasts. However, to our knowledge, only one study20 has analyzed possible mechanisms associated with post-exercise ambulatory blood pressure decreases. The authors demonstrated that an HIIE session resulted in a reduction in arterial stiffness as measured by pulse wave velocity during the waking period; however, data regarding changes in arterial stiffness after a single exercise session are limited and conflicting. Also, there is scarce evidence on the mechanisms associated with the reduction of ambulatory blood pressure, and therefore further investigations are needed to determine whether such adaptations occur in hypertensive patients.
In conclusion, the available evidence suggests that PEH after HIIE in most cases occurs due to a reduction in peripheral vascular resistance rather than a reduction in cardiac output. However, more information is needed to determine the real mechanisms associated with PEH in different clinical groups, as the mechanisms responsible may be different.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.