Post-cardiac injury syndrome (PCIS) is an inflammatory process involving the pericardium secondary to cardiac injury. It can develop after cardiac trauma, cardiac surgery, myocardial infarction, and, rarely, after certain intravascular procedures. We report a rare case of an iatrogenic cardiac rupture followed by PCIS with delayed inflammatory pericardial effusion after pacemaker implantation. A comprehensive literature review on this topic is provided.
A síndrome pós-lesão cardíaca (SPLC) corresponde a um processo inflamatório envolvendo o pericárdio, secundário à lesão cardíaca. Pode desenvolver-se após traumatismo cardíaco, cirurgia cardíaca, enfarte agudo do miocárdio, e, raramente, após alguns procedimentos intravasculares. Os autores apresentam o caso invulgar de uma rotura cardíaca iatrogénica após implantação de um pacemaker, seguida pelo desenvolvimento de um derrame pericárdico retardado, inflamatório, correspondendo a SPLC. A propósito do referido caso clínico, é efetuada uma revisão compreensiva da literatura acerca desta entidade.
Pacemaker implantation is a classic technique in cardiology. Materials have changed considerably in recent years, making the procedure safer and the indications broader. However, as in all invasive procedures, there is the risk of immediate and delayed intra and postoperative complications, such as system infection, lead displacement and post-cardiac injury syndrome (PCIS).
We report a rare case of an iatrogenic cardiac rupture followed by PCIS with delayed pericardial effusion after a pacemaker implantation. A comprehensive literature review on this topic is provided.
Case reportAn 89-year-old woman was admitted for syncope. She had a history of hypertension and dyslipidemia.
At admission, she reported a brief episode of loss of consciousness, without prodromes or head trauma and with spontaneous recovery. Her physical examination revealed bradycardia but no other relevant findings. The electrocardiogram showed advanced heart block with mean heart rate of 30 bpm. She was not under any negative chronotropic medication and laboratory tests showed no relevant electrolyte disturbances. Summary echocardiography revealed mild systolic dysfunction without pericardial effusion.
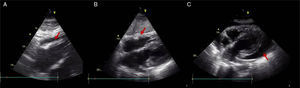
A temporary pacemaker was inserted via the right femoral vein, without immediate complications, followed by implantation of a dual chamber permanent pacemaker the next day. During this procedure, the patient presented a brief period of hypotension and pleuritic chest pain. The echocardiogram showed moderate systolic dysfunction and new moderate pericardial effusion (16 mm), with no signs of hemodynamic compromise (Figure 1). A diagnosis of iatrogenic right ventricle rupture was made and the patient was kept under clinical, electrical and echocardiographic monitoring. She presented progressive reduction of the pericardial effusion and was discharged by the 5th day, asymptomatic and without pericardial effusion (Figure 1).
Echocardiographic subcostal views: (A) after permanent pacemaker implantation revealing a new moderate pericardial effusion (arrow); (B) at hospital discharge, after a few days of clinical surveillance with no evidence of pericardial effusion (arrow); and (C) at readmission, with a large pericardial effusion and ‘swinging heart’ (arrow).
Four weeks later, she was readmitted for pleuritic chest pain and asthenia. Physical examination revealed reduced heart sounds with no other significant alterations, including in the pacemaker scar, which presented no inflammatory signs. The electrocardiogram showed sinus rhythm with P-wave synchronous ventricular pacing. Blood tests showed leukocytosis (17×109/l) and elevated C-reactive protein (CRP) (110 mg/dl), with no evidence of systemic infection or fever. Blood cultures were negative and the chest X-ray showed cardiomegaly without pleural effusion. The echocardiogram revealed a large pericardial effusion (25 mm), with ‘swinging heart’ and signs of hemodynamic compromise (inferior vena cava dilatation, mitral and tricuspid flow variation >50%, abnormal septal motion, mild diastolic compression of right heart chambers), suggestive of incipient tamponade physiology (Figure 1).
Pericardiocentesis was performed, with drainage of 350 ml of light yellow fluid, which was found to be a sterile exudate (Table 1). Post-procedural echocardiography still showed moderate pericardial effusion (18 mm), but with no signs of hemodynamic compromise. Autoimmunity study was negative. There was no sign of pacemaker dysfunction, sensing and pacing thresholds being optimal.
The patient was diagnosed with PCIS and medicated with aspirin (500 mg four times a day) and colchicine (1 mg twice a day). As there was no favorable clinical evolution or remission of the pericardial effusion, prednisolone was added on the seventh day (1 mg/kg/day), with eventual resolution of symptoms, laboratory parameters and pericardial effusion. She was discharged on the 15th day and remained asymptomatic at follow-up.
DiscussionPCIS is an inflammatory process involving the pleura (pleural effusion) and/or pericardium (pericarditis, pericardial effusion) secondary to cardiac injury.1 It can develop after cardiac trauma, cardiac surgery, myocardial infarction and certain intravascular procedures, including transvenous pacemaker lead insertion, electrophysiological studies and percutaneous coronary interventions, even in the absence of obvious cardiac perforation.1
Hence, the designation of PCIS covers post-myocardial infarction pericarditis (Dressler syndrome), post-pericardiotomy syndrome and post-traumatic pericarditis. In the setting of cardiac pacing, it appears to be a very rare condition, with only a few cases described in the literature.
Unlike acute myocardial rupture and acute pericarditis, which can both occur within 24 hours of transvenous pacing, PCIS typically occurs later on and represents an exclusion diagnosis.1 Although the precise cause is not clear, it appears to be due to an immune reaction precipitated by pacemaker-induced myocardial injury (late autoimmune or inflammatory response to pericardial irritation by minimally protruding electrodes, by low-grade bleeding with hemorrhagic pericarditis or by acute cardiac perforation). The underlying molecular mechanism may be similar to that of Dressler syndrome.5 There is no known predisposing underlying cardiac disease and the only demographic predictor seems to be advanced age.1 The potential role of technical factors (such as active fixation during lead positioning or previous temporary transvenous pacemaker use) in the development of PCIS has been postulated but needs further investigation.1,4
Clinical presentation includes pleuritic chest pain, shortness of breath and low-grade fever, symptoms that generally occur within the first month after the procedure.3 A rub (pericardial or pleural) may be detected and non-specific ECG changes can be found.2
On admission, elevated sedimentation rates and CRP levels, sometimes with both pericardial and pleural effusions, are typically documented. Laboratory analysis usually reveals exudative effusions.
Other documented laboratory alterations include unexplained falls in hemoglobin, low complement levels, presence of immune complexes in the pleural fluid and elevated anti-myocardial antibody levels in both serum and pleural effusion.6,7
Regarding differential diagnosis, clinical entities such as infectious pericardial effusion, viral pericarditis and delayed perforation must be ruled out. In this setting, clinical history, electrocardiography, pacemaker interrogation, cardiac imaging techniques and pericardial fluid analysis are crucial diagnostic tools. Previous history of viral infection and diffuse ST-segment elevation are highly suggestive of acute viral pericarditis. The presence of a light yellow pericardial effusion excludes delayed myocardial lead rupture. Echocardiography and computed tomography can show pericardial positioning of a pacemaker lead, which, as well as pacemaker dysfunction, suggests late cardiac rupture.8 Finally, pericardial effusion associated with infection of the pacemaker implantation site suggests a bacterial etiology and endocarditis must be ruled out.
If identified early, these patients will likely respond to NSAIDS, colchicine and/or prednisone, and medical treatment may eliminate the need for a surgical procedure.2 However, cases of large fluid volumes, recurrent fluid accumulation and/or tamponade physiology require pericardial fluid drainage and/or pericardial window at presentation.2
Regardless of the possible need for an invasive strategy in the acute phase, the outcome after PCIS secondary to pacemaker implantation is favorable, as all the reported cases presented a good evolution, even though there may be recurrence. However, delayed diagnosis and treatment of PCIS, as well as unnecessary tests to screen for other causes of pericardial or pleural effusion and fever, may prolong hospital stay and increase medical costs.3
In the reported case an iatrogenic cardiac rupture immediately after permanent pacemaker implantation was followed by a hemodynamically significant pericardial effusion requiring urgent pericardiocentesis. Bacterial infection of the pacemaker implantation site and infective endocarditis were excluded, as was delayed myocardial perforation. The clinical features, investigation and response to steroids indicated a diagnosis of PCIS.
This case highlights the need for a high suspicion index in the diagnosis of this rare entity after interventional procedures. Early recognition is crucial, both in order to proceed to the appropriate therapy and to prevent catastrophic complications, as well as to avoid prolonged hospital stay and increased medical costs.
ConclusionPCIS after pacemaker implantation is a rare situation with potentially serious complications. It constitutes an exclusion diagnosis in which a high suspicion index is necessary. The case described highlights the need to keep this entity in mind in patients who have undergone invasive cardiac procedures.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.