For years, the treatment of high-risk pulmonary embolism (PE) was based on two well-defined strategies: thrombolysis, whose benefits have been documented in randomized trials, and surgical embolectomy. However, mechanical reperfusion by percutaneous techniques is used in an increasing number of patients, and is a valid therapeutic option when there is a formal contraindication to thrombolysis, as rescue therapy when thrombolysis fails to improve hemodynamics, and/or when emergency surgical thrombectomy is unavailable or contraindicated.
This article discusses the indications for the use of percutaneous techniques in PE, reports the initial experience of our center with the AngioJet® thrombectomy device (Possis Medical Inc., Minneapolis, MN, USA) and reviews the available evidence, the most recent recommendations and the main complications associated with this procedure.
A terapêutica no tromboembolismo pulmonar (TEP) de alto risco baseou-se durante anos em duas estratégias bem definidas: a trombólise, cujos benefícios foram documentados em estudos aleatorizados, e a embolectomia cirúrgica. Contudo, a reperfusão mecânica por técnicas percutâneas tem sido utilizada num número crescente de doentes. Estas técnicas constituem uma alternativa terapêutica válida na presença de contraindicação formal para trombólise, como terapêutica de recurso quando a trombólise é ineficaz, ou como alternativa à embolectomia cirúrgica na ausência de disponibilidade da técnica ou na presença de contraindicação para a mesma.
Neste artigo pretendemos discutir as indicações para a utilização das técnicas percutâneas no TEP e apresentar a experiência inicial do nosso centro com o sistema de trombectomia por cateter AngioJet® (Possis Medical Inc, Minneapolis, MN, EUA). Foi intenção dos autores rever a evidência disponível, as mais recentes recomendações para a sua utilização, assim como as principais complicações associadas a este procedimento.
Pulmonary embolism (PE) is a common diagnosis associated with high acute mortality, estimated at 7–11% in prospective studies.1 High-risk PE is defined as the presence of shock or persistent arterial hypotension (systolic blood pressure <90 mmHg or a pressure drop of ≥40 mmHg for >15 min if not caused by new-onset arrhythmia, hypovolemia or sepsis), and represents an emergency requiring specific management.2,3
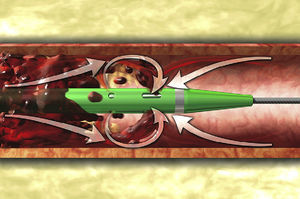
In the presence of absolute contraindications or an inadequate response to thrombolysis, for years treatment consisted of surgical embolectomy. However, this is not always immediately available, and in recent years percutaneous techniques have been used in an increasing number of patients. Currently available techniques for recanalizing the pulmonary arteries can be classified into four types: (1) suction thrombectomy, (2) fragmentation and distal dispersion, (3) rotational thrombectomy, and (4) rheolytic thrombectomy (RT).4 The latter uses a high-pressure saline jet that generates a pressure gradient by Bernoulli's principle, for lysis and removal of the thrombus (Figure 1).5 Such systems are also capable of pharmacomechanical therapy, injecting a low dose of a thrombolytic agent into the thrombus using a power-pulse spray. A combined technique, ultrasound-accelerated thrombolysis, has recently been evaluated. This aims to facilitate thrombolysis and thus permit the administration of lower doses of fibrinolytics.6,7
Diagram of the AngioJet® catheter with its dual lumen, an intake lumen through which a high-pressure saline jet is pumped and an exhaust lumen through which the thrombus is removed. Three orifices in the catheter tip enable three high-pressure jets to form towards the exhaust lumen, which creates a pressure gradient to fragment and aspirate the thrombus.
Various series have shown good results using these new techniques. However, they have never been assessed in randomized clinical trials, and so doubts remain as to their efficacy and safety.
This article describes the initial experience of our center with RT using the AngioJet® system (Possis Medical Inc., Minneapolis, MN, USA) and presents a review of the literature.
Case presentationCase 1A 47-year-old man, with a history of cerebral arteriovenous malformation (AVM) treated by radiosurgery, was admitted to the neurosurgical ward with right temporo-occipital intraparenchymal hemorrhage extending into the ventricular system for conservative treatment. On the 14th day after admission he presented sudden-onset severe respiratory failure and shock. Thoracic computed tomography (CT) angiography confirmed the suspicion of bilateral PE, on the left with a saddle thrombus from the pulmonary artery bifurcation to the lobar and segmental branches of the upper and lower left lobes, and on the right with involvement of the upper lobe artery and segmental branches, the interlobar artery and the lobar and segmental branches of the middle and lower lobes. Transthoracic echocardiography (TTE) showed marked right ventricular (RV) dilatation, mild tricuspid regurgitation and pulmonary flow suggestive of pulmonary hypertension (PH). The setting was interpreted as high-risk PE and the patient was transferred to the intensive care unit (ICU). In view of the patient's shock and absolute contraindication for intravenous thrombolysis, it was decided to perform RT and an inferior vena cava filter was placed. However, at the start of the procedure he suffered cardiopulmonary arrest (CPA) with asystole, reverted after four cycles of advanced life support (ALS) and thrombectomy, which resulted in immediate hemodynamic improvement and slight angiographic improvement. Inotropic and ventilatory support were withdrawn after four days, low molecular weight heparin was begun on the fifth day, and craniotomy and removal of the AVM were performed two months later. Three months after the acute event, thoracic CT angiography showed complete resolution of the intraluminal thrombi.
Case 2A 78-year-old woman was admitted to the emergency room with shock, respiratory failure and impaired consciousness. She had undergone colorectal surgery 15 days previously. There was no visible blood loss, although she had had severe rectal bleeding in the previous week. Given the absence of blood pressure response to fluid therapy, elevated troponin T and severe RV dilatation and functional impairment on TTE, it was decided to perform thoracic CT angiography, which revealed bilateral central PE, with subtraction images suggestive of multiple thrombi in the main right and left pulmonary arteries and all the lobar and segmental branches, causing significant luminal obstruction, particularly of the lower lobe arteries.
Although there was formal indication for intravenous thrombolysis, the patient was considered to be at high bleeding risk, and it was decided to perform percutaneous mechanical reperfusion with the AngioJet® system. During the procedure she presented brief self-limited episodes of respiratory arrest and extreme bradycardia, followed by hemodynamic stabilization, withdrawal of vasopressor support and angiographic improvement.
However, two hours after the procedure the patient again suffered shock refractory to fluid therapy and inotropic support, with persisting RV dilatation. Given probable rethrombosis, life-saving thrombolysis was performed with alteplase 100 mg over two hours and non-fractionated heparin was administered. Improvement was seen in hemodynamics and gas exchange, but various bleeding complications ensued, requiring blood transfusion and leading to multiple organ dysfunction necessitating renal replacement therapy. Invasive ventilation was not required. Vasopressor support was discontinued after four days and dialysis after 10 days. Repeat TTE on the 11th day showed normal-sized right cardiac chambers, but pulmonary flow still suggested PH. She was discharged after 58 days.
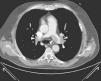
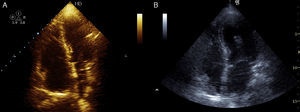
Case 3A 47-year-old woman, obese and with peripheral venous insufficiency, was admitted to the emergency room with syncope, sudden-onset dyspnea, epigastric pain, severe respiratory failure and shock. She had begun taking oral contraceptives three days previously. A few minutes after admission she suffered CPA with pulseless electrical activity and ALS was begun. Spontaneous return of circulation occurred several times but was immediately followed by CPA. TTE showed dilatation of the right chambers (Figure 3A), grade III/IV tricuspid regurgitation (pulmonary artery systolic pressure 45 mmHg above central venous pressure), pulmonary flow suggestive of PH and impaired RV systolic function.
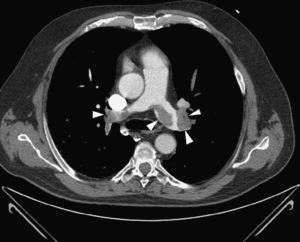
Given the suspicion of obstructive shock due to high-risk PE, intravenous thrombolysis was performed with a 50-mg bolus of alteplase, which resulted in spontaneous permanent return of circulation after around 45 min of ALS. Thoracic CT angiography showed thromboembolic foci in the distal portion of both pulmonary arteries, in the origin of several lobar arteries, and most noticeably in the segmental branches of the right lower lobe artery (Figure 2). As the setting of shock remained refractory to supportive measures, it was decided to perform RT using the AngioJet® system, which resulted in immediate hemodynamic improvement and partial angiographic improvement. The patient remained under ventilatory and inotropic support for 10 days, followed by a favorable clinical course and complete neurological recovery. TTE performed one month after the acute event showed no RV dilatation or dysfunction or signs of PH (Figure 3B). The patient was discharged after 37 days.
Despite advances in the diagnosis and treatment of high-risk PE, acute mortality remains high and can exceed 50%.3,8,9 Although a few randomized trials have shown that thrombolysis is the only intervention that reduces mortality and rethrombosis, in the International Cooperative Pulmonary Embolism Registry (ICOPER), thrombolysis did not reduce 3-month mortality or rethrombosis.8 A meta-analysis in 2004 by Wan et al. of 11 randomized trials comparing thrombolytic therapy with heparin showed a tendency for better clinical outcomes with thrombolysis, but in a subanalysis of five of these trials that included patients with hemodynamic instability, there was a significant reduction in mortality and rethrombosis with thrombolysis compared to heparin only (9.4% vs. 19%; number needed to treat=10).10 Although there have been no randomized trials specifically assessing thrombolysis in the context of CPA and high clinical suspicion of PE, it is accepted as a possible therapeutic option.11,12 However, the rate of bleeding complications associated with thrombolysis is high, with an incidence of 13–21% of major bleeding and of 1.8–3% of intracranial and/or fatal bleeding in the literature reviewed.8,10,13 Despite the short-term benefits of thrombolysis in hemodynamically unstable PE, some authors suggest that in some patients there is only partial clearance of the obstruction followed by distal embolization, resulting in PH and increased long-term mortality. A 2003 study by Meneveau et al. of 249 patients with PE receiving thrombolysis showed that survival among those who survived the acute phase was only 56% at 10 years, and identified residual vascular pulmonary obstruction of >30% post-thrombolysis as an independent predictor of poor long-term prognosis.14
Surgical embolectomy is usually reserved for patients requiring cardiopulmonary resuscitation, when there is absolute contraindication to thrombolysis, as a rescue treatment when there is no response to intensive medical and thrombolytic therapy, and in those with patent foramen ovale and intracardiac thrombi.2 The results of initial studies were disappointing, with early mortality of 20–50%, but patients referred for the procedure tended to have a very poor prognosis from the outset.15–18 Following recent advances in diagnosis, surgical techniques and postoperative care, and with wider indications for surgery, which is no longer performed only in the context of severe shock, early mortality rates as low as 6% have been reported.19 The timing of surgery is also now given greater importance: in a small retrospective study of 15 patients with high-risk PE, of the 10 who underwent emergent embolectomy within 24 hours, no deaths or significant complications were recorded, while mortality in those treated after 24 hours was 60%.20
There have been few studies comparing surgical embolectomy with thrombolysis, all of them retrospective. Gulba et al. observed lower mortality (23% vs. 33%), fewer major bleeding complications (15% vs. 25%) and a lower rate of rethrombosis (7.7 vs. 21%) with surgical embolectomy,21 while Meneveau et al. also found that embolectomy following failed thrombolysis was more effective than repeat thrombolysis, but mortality in the surgical group was still high.22,23 A major limitation of surgical embolectomy, which probably explains its underuse, is the lack of availability of the technique and the need for highly trained surgical teams.
Percutaneous treatment of high-risk PE has also evolved. According to the current European Society of Cardiology guidelines and the 2011 American Heart Association Scientific Statement, catheter-based interventions can be performed as an alternative to thrombolysis when there are absolute contraindications, as adjunctive therapy when thrombolysis has failed to improve hemodynamics, or as an alternative to surgery if the latter is unavailable or contraindicated.2,24 The American College of Chest Physicians guidelines also recommend catheter-based thrombus removal in patients with shock that is likely to cause death before systemic thrombolysis can take effect (class IIa recommendation, level of evidence C).25
Percutaneous techniques can be classified into three types: (1) mechanical perfusion, the preferred approach in patients with absolute contraindication to thrombolysis; (2) catheter-directed local thrombolysis; and (3) pharmacomechanical or combined, including ultrasound-accelerated thrombolysis. The latter two are often used when there is relative contraindication to systemic thrombolysis, since local thrombolysis carries a lower risk of bleeding complications.4,26
The evidence on catheter-based interventions is limited to case reports, retrospective analyses of small series and systematic reviews; there have been no randomized clinical trials comparing percutaneous treatment with systemic thrombolysis. A systematic review by Shaft et al. that included older techniques such as suction thrombectomy with the Greenfield catheter assessed the clinical success of catheter-tip devices in patients with high-risk PE. The success rate of mechanical reperfusion alone was 81%, and 95% when combined with local infusion of thrombolytics.26 A more recent systematic review and meta-analysis of six prospective and 29 retrospective studies with a total of 594 patients assessed the effectiveness of catheter-directed therapy using modern low-profile devices (≤10F), including RT with the AngioJet® system, with or without local thrombolytic infusion. Clinical success, defined as stabilization of hemodynamics, resolution of hypoxia, and survival, was achieved in at 86%, while the rate of major procedural complications was 2%. Local injection of thrombolytics without previous intravenous thrombolysis was performed in 95% of patients. The effect of catheter-based mechanical reperfusion could not be assessed in isolation, since 67% of patients underwent combined therapy.27
Various series have specifically assessed RT with the AngioJet® system, including a retrospective study by Chechi et al., which included 51 patients with high- and intermediate-risk PE and substantial involvement of the pulmonary vascular bed on CT. Technical success, defined as a significant improvement in obstruction, perfusion and Miller index, was obtained in 92% of patients. Four patients had major bleeding and eight (15.7%) died in hospital. No event-related deaths were reported in long-term follow-up (35.5±21.7 months).28 The safety and effectiveness of the technique, as well as good long-term clinical outcomes, were also demonstrated in a cohort of 25 patients by Margheri et al.29
In all the series and case reports reviewed, RT with or without local thrombolysis invariably showed good clinical results and relatively low mortality in centers with experienced operators.30–33
As pointed out above, the evidence indicates that combined (pharmacomechanical) therapy is more effective than mechanical thrombectomy alone, although the latter remains the preferred approach in patients with absolute contraindication to thrombolysis. The question remains as to whether local catheter-directed thrombolysis is superior to combined therapy in patients with no or only relative contraindication to thrombolysis.4 Two studies in our review compared local catheter-directed thrombolysis with a pharmacomechanical approach. A small retrospective analysis compared the efficacy of local thrombolysis with RT (AngioJet®) associated with local infusion of low-dose thrombolytics. Despite the lower doses of thrombolytics, pharmacomechanical therapy was associated with more rapid hemodynamic recovery.4,34 In 2009, in a cohort of 33 patients, Lin et al. showed that ultrasound-accelerated thrombolysis was more effective and was associated with lower doses of thrombolytics and fewer bleeding complications than catheter-directed thrombolysis.4,35
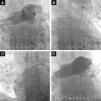
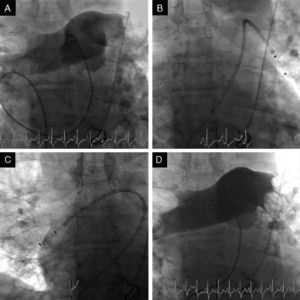
Two of our patients were referred due to contraindication to thrombolysis and the other for failure of thrombolysis to improve hemodynamics. At the time of the procedure two patients were sedated and ventilated, and all were under inotropic support. The right femoral vein was punctured and a 7F introducer was inserted, followed by arteriography of the pulmonary artery trunk and selective arteriography of the right and left pulmonary arteries using a 6F angled pigtail catheter. A 0.035″ hydrophilic guidewire was then positioned in the affected pulmonary artery and the 6F dual-lumen AngioJet® catheter was advanced. The catheter was activated proximally to distally, with one or two complete passes. The procedure was repeated for the affected lobar arteries and for the contralateral pulmonary artery if necessary. An angiographic review was performed at the end of the procedure (Figure 4). The intervention should be halted as soon as hemodynamic recovery is confirmed or if the total activation time recommended by the manufacturer is reached, irrespective of the final angiographic result. In the last patient, it was decided to implant a temporary transvenous pacemaker by a femoral route at the beginning of the procedure. Following the procedure, all patients were transferred to the ICU.
Fluoroscopic images during initial arteriography of the pulmonary trunk (more selective for the right pulmonary artery), showing massive bilateral pulmonary embolism with thrombi in the main pulmonary arteries and involving all the lobar arteries, with significant flow obstruction (A); 6F AngioJet® catheter positioned in the left pulmonary artery (B) and in the right pulmonary artery (C); final image, demonstrating slight angiographic improvement but with immediate improvement in hemodynamics and gas exchange (D).
As well as complications related to vascular access, contrast reactions and anticoagulation, there are complications specifically related to percutaneous techniques, particularly the risk of perforation leading to hemoptysis or tamponade, pulmonary infarction, and reperfusion syndrome with alveolar hemorrhage. The AngioJet® system is particularly associated with transient bradyarrhythmias and asystole, as seen in our initial experience, due to hemolysis and the resulting release of potassium, adenosine and bradykinin. This risk is increased when thrombectomy lasts longer than 20 s, but can be minimized by activating the AngioJet® intermittently.36 Total activation time should not exceed 3 min for unilateral PE and 4 min for bilateral PE (2 min per lung). Hemoglobinuria is common and should not be confused with hematuria.
Teamwork is essential to minimize complications. The team should include an interventional cardiologist with experience in this area, an anesthetist and an internist (who are responsible for the initial assessment and referral of the patient), and an intensivist or cardiologist-intensivist able to deal with periprocedural complications.
ConclusionThe three cases presented are examples of the application and results of current percutaneous techniques for the treatment of high-risk PE, which may even be considered first-line options in selected patients. Our initial experience of RT with the AngioJet® catheter is supported by the available evidence, which although still sparse indicates that the technique has a good success rate, with an acceptable risk of complications and relatively low mortality considering that these are critical patients with a poor prognosis. In the light of current knowledge, our center is about to establish a protocol that includes concomitant local infusion of thrombolytics in selected patients without absolute contraindication to thrombolysis.
In the absence of controlled trials directly comparing different therapeutic options, the best strategy should be decided case by case by a multidisciplinary team, always bearing in mind the factors specific to each patient, the availability of different therapeutic options and the center's experience.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Faria R, Oliveira M, Ponte M, et al. Trombectomia percutânea reolítica no tratamento de tromboembolismo pulmonar de alto risco: experiência inicial de um centro. Rev Port Cardiol. 2014;33:371–377.