Pseudoaneurysm of the ascending aorta is a rare complication, usually after thoracic surgery or trauma.
Since surgical repair is associated with very high morbidity and mortality, percutaneous closure has been described as an alternative.
In this regard, we present a case in which a symptomatic large pseudoaneurysm of the ascending aorta was treated percutaneously due to the high surgical risk.
Despite the technical difficulties, this procedure had a good final result followed by clinical success.
O pseudoaneurisma da aorta ascendente consiste numa complicação rara, habitualmente na sequência de cirurgia cardiotorácica ou traumatismo.
Dado que a reparação cirúrgica do mesmo se associa a uma elevada morbimortalidade, o encerramento percutâneo tem vindo a ser descrito como uma alternativa viável.
Neste contexto, apresentamos um caso caracterizado por um volumoso e sintomático pseudoaneurisma da aorta ascendente, o qual fora submetido a tratamento percutâneo, devido ao elevado risco cirúrgico.
Apesar das dificuldades do ponto de vista técnico, este procedimento obteve um bom resultado final, com sucesso em termos clínicos.
Pseudoaneurysm of the ascending aorta is a relatively rare but serious complication that usually develops following thoracic surgery, including aortic valve replacement, coronary artery bypass grafting, aortic dissection repair and orthotopic cardiac transplantation.1–5 The incidence of this complication following aortic surgery can reach 23% at 15 years after surgery. Other potential etiologies include endocarditis and thoracic trauma.6
Clinical presentation ranges from completely asymptomatic for years to symptoms related to the mass effect on surrounding structures.6
If left untreated, aortic pseudoaneurysms can evolve to rupture, thrombosis, distal embolization and fistula formation, with high mortality (up to 61%).6
Surgical repair of this complication is the conventional treatment, but it is associated with very high morbidity and mortality (mortality can reach 46%) and in some cases is not even feasible (due to the technical difficulties in patients who have undergone prior complex thoracic surgeries), so percutaneous closure has been described as an alternative.1–6
In the past, several percutaneous techniques for closure of a pseudoaneurysm have been attempted such as stent grafting, coil implantation or thrombin injection, but the final results obtained were suboptimal. In recent years, different types of devices have occasionally been used with success in off-label indications. The first percutaneous closure was published in 2005, but experience since then has been limited, usually to patients not suitable for surgery or with high surgical risk. The latest case was published in 2014, with a good final result and without complications.2–4,6 Overall, the success rate for percutaneous closure is 80%, with a 12% device embolization rate and an 8% failure rate of the transcatheter technique to close the pseudoaneurysm, necessitating conversion to surgical closure.5,6
We present a case in which a symptomatic large pseudoaneurysm of the ascending aorta was treated percutaneously due to the patient's high surgical risk.
Case reportAn 81-year-old man with a history of aortic bioprosthesis implanted due to severe aortic regurgitation, coronary artery disease (treated by percutaneous transluminal coronary angioplasty of the mid left anterior descending artery), arterial hypertension and dyslipidemia, was evaluated for atypical thoracic pain, after an asymptomatic period of two years post surgery. The patient was medicated with aspirin 100 mg daily, lisinopril 20 mg daily, carvedilol 6.25 mg twice daily, omeprazole 20 mg daily and rosuvastatin 10 mg, with good control of vascular risk factors.
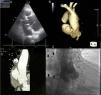
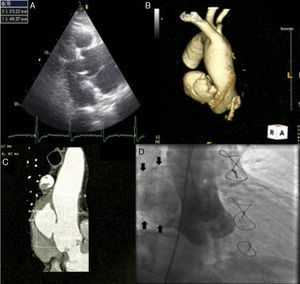
During complementary investigation, a chest X-ray showed widening of the mediastinal silhouette and a transthoracic echocardiogram revealed a pseudoaneurysm of the ascending aorta, 35 mm above the aortic valve (Figure 1A).
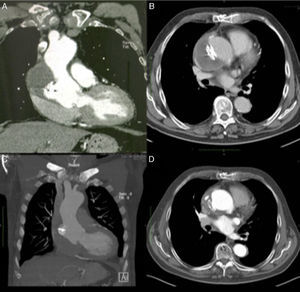
Imaging of ascending aortic pseudoaneurysm. (A) Transthoracic echocardiography showing ascending aortic pseudoaneurysm in parasternal view; (B) three-dimensional computed tomography (CT) reconstruction of aorta; (C) CT scan with intravenous contrast showing the dimensions of the neck and cavity of the pseudoaneurysm; (D) aortography with contrast opacification of pseudoaneurysm cavity (solid arrows).
Coronary computed tomography angiography (CCTA) (Figure 1B and 1C) and aortography (Figure 1D) confirmed a large pseudoaneurysm (98 mm×48 mm) and a defect in the aortic wall (19 mm×19 mm).
The patient was considered to be at high surgical risk (EuroSCORE II of 12.21%) and was referred for percutaneous treatment (closure of the pseudoaneurysm with a non-dedicated device).
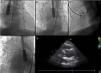
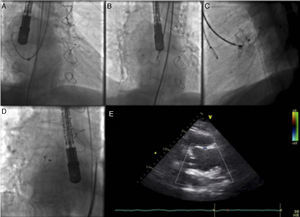
First of all, an attempt was made with a 9F Amplatzer® TorqueVue® delivery sheath through a right femoral artery approach. The pseudoaneurysm was engaged with a standard 0.035 wire and a left internal mammary diagnostic catheter inside the delivery sheath, but this was too short and did not enter the pseudoaneurysm cavity (Figure 2A). The sheath was then changed to a 12 F St. Jude®/Daig® sheath that successfully entered the pseudoaneurysm. A 20 mm Amplatzer® atrial septal defect (ASD) occluder was used, although considerable resistance was encountered navigating the device and it was impossible to proceed behind the aortic arch. An 8F Judkins Right guide catheter, with its proximal tip cut, was placed inside the delivery sheath for extra support, in a mother-child technique, enabling the device to navigate distally in the sheath. At first, the proximal disk did not conform to its original configuration (Figure 2B), but after a series of careful retrieval and reposition maneuvers the device finally acquired a stable position (Figure 2C–E).
Images illustrating percutaneous closure of aortic pseudoaneurysm. (A) Left internal mammary diagnostic catheter inside the delivery sheath engaging the pseudoaneurysm cavity; (B) Amplatzer atrial septal defect (ASD) occluder with the distal disk opened inside the pseudoaneurysm cavity, and the proximal disk misshapen inside the aortic lumen; (C) Amplatzer ASD device deployed across the aortic wall defect; (D) Amplatzer ASD device released in a stable position; (E) transthoracic echocardiography showing position of the device without interfering with the bioprosthesis.
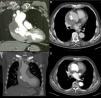
CCTA at one month (Figure 3A and B) and 12 months (Figure 3C and D) after the procedure showed the device correctly positioned and complete closure of the pseudoaneurysm with thrombosis of its cavity.
Evolution over time of ascending aortic pseudoaneurysm closure. (A and B) Computed tomography (CT) scan at one month showing Amplatzer ASD device in a stable position and organizing thrombus inside the pseudoaneurysm cavity; (C and D) CT scan at one year with device maintaining its position and partial resolution of the excluded pseudoaneurysm cavity.
After 14 months of follow-up the patient remains asymptomatic and without any events.
Discussion and conclusionThis case report illustrates a relatively rare complication of thoracic surgery, namely an ascending aorta pseudoaneurysm.
Although surgical repair remains the definitive therapy, in view of the patient's high perioperative risk he was treated by percutaneous closure of the pseudoaneurysm, which has a high (80%) success rate, as described in the literature.2–6
Experience with percutaneous closure of aortic pseudoaneurysms has increased steadily since the introduction of the technique in 2005, with several benefits, including fewer potential complications and avoidance of surgical risk.6
In this case, despite all the technical difficulties, the procedure had both imaging and clinical success, rendering the patient asymptomatic and without events in a follow-up of over one year.
Consequently, this result confirms that percutaneous closure with a non-dedicated device is an effective and safe alternative to surgery as a definitive treatment in aortic pseudoaneurysms.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.