Mitral stenosis (MS) is frequently associated with the development of atrial fibrillation (AF) as a consequence of hemodynamic and inflammatory changes in the left atrium. Both conditions predispose to thrombus formation, with frequent involvement of the left atrial appendage (LAA), and consequent increase in the incidence of systemic thromboembolic events. Percutaneous mitral valvuloplasty (PMV) reduces the risk of thromboembolism in patients with significant mitral stenosis. Percutaneous LAA closure is also associated with a reduction in thromboembolic risk in patients with AF, but there are no data regarding the use of this technique in patients with significant mitral valve disease. We report the case of a 57-year-old-woman with significant MS and permanent AF, in New York Heart Association functional class II, who despite adequate oral anticoagulation with acenocoumarol, presented several clinical episodes of systemic thromboembolism in the last four years. It was decided to perform a combined percutaneous procedure, including both PMV and percutaneous LAA closure with the Amplatzer Cardiac Plug device. No significant acute complications occurred and the patient was discharged on indefinite treatment with acenocoumarol associated with aspirin 100 mg/d for three months. After a one-year follow-up, there have been no new embolic episodes or other complications.
A estenose mitral (EM) é frequentemente associada ao desenvolvimento de fibrilhação auricular (FA) como consequência de alterações hemodinâmicas e inflamatórias na aurícula esquerda. Ambas as condições predispõem à formação de trombos, com frequente envolvimento do apêndice auricular, e consequente aumento da incidência de eventos tromboembólicos sistémicos. A valvuloplastia mitral percutânea (VMP) reduz o risco de tromboembolismo em doentes com EM significativa. O encerramento percutâneo do apêndice auricular esquerdo (PCLAA) na FA também está associado a uma redução de risco de tromboembolismo em pacientes com FA, mas não existem dados sobre a utilização desta técnica em doentes com MS. Relatamos o caso de uma mulher de 57 anos de idade com significativa EM e FA permanente, em classe funcional II, que, apesar de anticoagulação oral adequada com acenocumarol, apresentou nos últimos quatro anos vários episódios clínicos de tromboembolismo sistémico. Foi decidido realizar um procedimento percutâneo combinado de PMV e PCLAA com dispositivo Amplatzer Cardiac Plug. Não se verificaram complicações agudas significativas e o paciente recebeu alta com acenocumarol de forma indefinida, associado com aspirina 100 mg/d durante três meses. Depois de um ano de follow-up, não houve novos episódios embólicos ou outras complicações.
Mitral stenosis (MS) is associated with increased thromboembolic risk, especially in individuals with atrial fibrillation (AF), a common finding in these patients.1,2 Percutaneous mitral valvuloplasty (PMV) in patients with hemodynamically significant MS is associated with a reduction in thromboembolic risk.3 Also, percutaneous left atrial appendage (LAA) closure is an emerging procedure that may be considered in patients with AF with high thromboembolic risk and in whom chronic oral anticoagulation is contraindicated.4–6
We describe the case of a patient with significant MS and a history of repeated thromboembolic events in spite of oral anticoagulation with acenocoumarol who underwent both PMV and percutaneous LAA closure in the same procedure. Synergies between the two procedures are discussed.
Case reportA 57-year-old woman with significant MS, in New York Heart Association functional class II/IV, was referred to our unit for PMV, as echocardiographic parameters were suitable (mitral area 1.1 cm2, mild mitral regurgitation, Wilkins score 7). Interestingly, she had previously undergone PMV 12 years before.
The patient had permanent AF and was treated chronically with acenocoumarol. Despite adequate oral anticoagulation, in the last four years she had presented at least two episodes of ischemic stroke: an embolic stroke two years previously in the right middle cerebral artery territory, and an embolic stroke eight months previously in the right posterior inferior cerebellar artery which caused transtentorial herniation. After these episodes the patient presented an acceptable neurological recovery and was independent for everyday activities. One month before admission, she had presented a non-ST-segment elevation acute myocardial infarction. Coronary computed tomography showed no coronary stenosis. Coronary embolism was diagnosed as the probable cause of the ischemic event and the patient had no recurrent ischemia or other complications. Our center's heart team decided to perform PMV and percutaneous LAA closure during the same procedure.
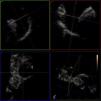
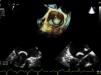
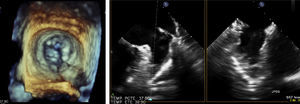
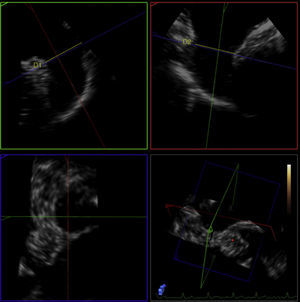
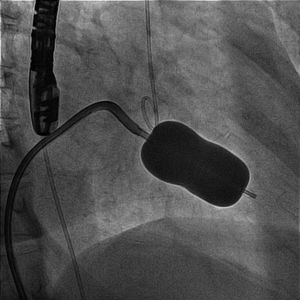
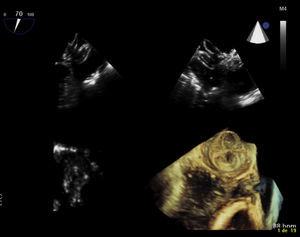
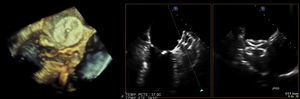
Transesophageal echocardiography performed 24 hours prior to the procedure showed no thrombus in the left appendage but revealed the presence of high-density spontaneous contrast (Figure 1). The LAA showed a 29 mm×26 mm entry orifice (Figure 2).
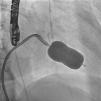
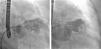
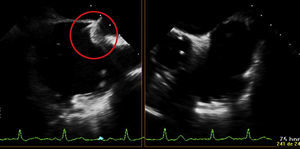
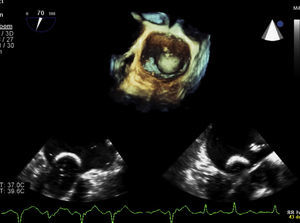
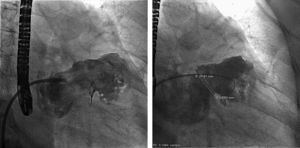
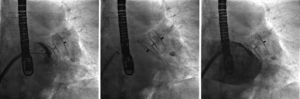
In the procedure, the middle portion of the interatrial septum was punctured (Figure 3) and PMV was performed first by the usual technique with a Mullins sheath and a Brockenbrough needle and inflation of a 26 mm Inoue balloon (Figures 4 and 5), with a good result (absence of transmitral gradient, 2.1 cm2 mitral area and no increase in the degree of valvular regurgitation). Percutaneous LAA closure was then performed. Keeping the guidewire in the same transseptal puncture, a pigtail catheter was introduced into the LAA to allow selective injections into this structure (Figure 6). A 30 mm Amplatzer Cardiac Plug (ACP) (St. Jude Medical), specific for percutaneous LAA closure, was inserted via the ACP release sheath and implanted in the left appendage. It was released successfully and without complications (Figures 7 and 8).
The patient was discharged on indefinite treatment with acenocoumarol, associated with aspirin (100 mg/d) for three months. After one year follow-up there have been no further embolic episodes or other complications (Figure 9).
DiscussionPMV is a common procedure in patients with significant MS, poor functional class and echocardiographic parameters suitable for the technique. The procedure also shows good results in patients who have previously undergone PMV, as in the case of our patient.2 What makes the case described interesting is the fact that the patient had presented numerous cardioembolic episodes in spite of adequate chronic oral anticoagulation, so it was decided to undertake a combined PMV and percutaneous LAA closure procedure to reduce the risk of post-PMV embolic recurrence. Since a number of the potential complications of percutaneous LAA closure are associated with transseptal puncture, also used for PMV, the risk of performing the two procedures together is probably lower than if they are done separately. There are technical considerations related to the site of the transseptal puncture, since the ideal position differs according to the type of procedure. In our case the puncture was performed in the mid portion of the septum, allowing a relatively easy procedure.
Significant MS increases pressure and alters flow in the left atrium. This is associated with an increased incidence of AF and hypercoagulability, both explaining the high rate of embolism in patients with this valvulopathy.3 PMV has been shown to reduce the incidence of systemic embolism in MS, and PMV is therefore recommended even in patients with significant asymptomatic MS and high thromboembolic risk.2 In the case presented, the therapeutic strategy adopted was intended not only to improve functional class, but also to reduce the risk of recurrent embolism by the double mechanism of improving hemodynamic parameters in the left atrium and excluding the LAA.
Percutaneous LAA closure is becoming increasingly common in patients with AF, high embolic risk and contraindications for anticoagulation, but very few data are available regarding this procedure in patients with rheumatic AF. The guidelines recommend LAA closure during surgical MS procedures, as it is associated with a significant reduction in risk of postoperative embolic events.7–9 Similarly, some patients undergoing PMV may also benefit from percutaneous LAA closure performed during the same operation.
Our patient was discharged under chronic oral anticoagulation, since LAA closure does not completely eliminate the risk of embolism in MS patients and she had no contraindications for this treatment. Out of 136 patients who underwent atrial appendage closure during a mitral valve operation, Almahameed et al.8 observed that thromboembolic events were more frequent in patients whose anticoagulant treatment had been stopped after 3.5 years of treatment (10% vs. 15%). The ideal anticoagulant treatment after intracavitary device implantation is unclear, suggesting the use of aspirin in our patient in the three months following the operation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.