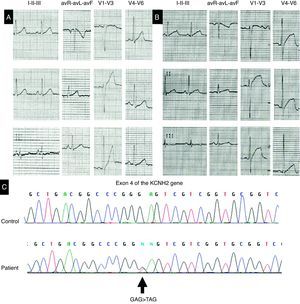
A 37-year-old man was admitted to our department after an episode of rapid regular palpitations, triggered by emotional stress. He had no previous symptoms and was not taking any medication. There was no relevant family history. The first two electrocardiograms documented sinus rhythm and a pattern of abnormal repolarization with ST-segment elevation. The corrected QT interval (QTc) was between 428 and 468 ms (Figure 1A and B). Laboratory tests showed no abnormalities and exercise testing was normal. Holter monitoring documented intermittent QTc prolongation (maximum 580 ms), with no other abnormalities. Screening for mutations in the KCNQ1, KCNH2, SCN5A and KCNE1 genes for LQT1, LQT2, LQT3 and LQT5 variants of long QT syndrome (LQTS) revealed a c.529G>T (p.Glu177X) mutation in heterozygosity in the KCNH2 gene of LQT2 (Figure 1C). This variant has not been previously reported, but, since it produces a premature STOP codon, there is a high probability that it is actually pathogenic. The patient was discharged home on beta-blocker therapy and with information on drugs that prolong QT, to be avoided. Clinical and molecular study of first-degree relatives is currently under way.
LQTS patients can have intermittent QT prolongation, but they are at risk for ventricular arrhythmias that can be triggered by various stimuli and by drugs that prolong QT.
Most drugs that prolong QT act by blocking the Ikr ionic current, encoded by the KCNH2 gene. In our patient, intermittent QT prolongation was documented and a novel KCNH2 mutation was identified, enabling arrhythmia prevention therapy to be initiated.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.