Refractory angina is defined as persistent angina (≥3 months) despite optimal medical and interventional therapies. It is increasing in frequency, due to the success of current medical and interventional therapies in improving the prognosis of coronary artery disease. Long-term mortality is similar to that of patients with asymptomatic stable disease, but it affects patients’ quality of life, and has a significant impact on health care resources.
Several therapeutic targets have been investigated, most with disappointing results. Many of the techniques have been abandoned because of lack of efficacy, safety issues, or economic and logistic limitations to wider applicability.
The primary focus of this review is the coronary sinus Reducer, supporting evidence for which, although scarce, is promising regarding safety and efficacy in improving anginal symptoms and quality of life. It is also accessible to virtually all interventional cardiology departments.
A angina refratária define‐se como a persistência de sintomas superior a três meses apesar da terapêutica médica aprimorada e revascularização. É uma entidade em crescimento, resultado da melhoria do prognóstico da doença coronária com a terapêutica farmacológica e com as técnicas de revascularização contemporâneas. A mortalidade em longo prazo enquadra‐se no espetro prognóstico da doença estável assintomática, contudo interfere com a qualidade de vida do doente e tem um impacto significativo nos sistemas de saúde.
Múltiplos alvos terapêuticos têm sido investigados. Contudo, a maioria com resultados dececionantes. Muitas das técnicas foram abandonadas por ausência de eficácia, problemas de segurança e limitações tanto logísticas como económicas à sua implantação.
Esta revisão incide essencialmente sobre o dispositivo de redução do seio coronário, cuja evidência, embora ainda escassa, é promissora relativamente à segurança e eficácia na redução dos sintomas anginosos e na melhoria da qualidade de vida. Para além do seu efeito terapêutico, é uma opção virtualmente acessível a todos os serviços de cardiologia de intervenção.
Refractory angina is defined in the 2019 European Society of Cardiology (ESC) guidelines on chronic coronary syndromes as long-lasting symptoms (≥3 months) due to established reversible ischemia in the presence of obstructive coronary artery disease, which cannot be controlled by medical therapy with the use of second- and third-line pharmacological agents, coronary artery bypass grafting (CABG), or percutaneous coronary intervention (PCI) of chronic total coronary occlusions.1 However, this classification excludes patients with long-standing symptoms and ischemia but without obstructive disease, and so many researchers have adopted a wider definition based on the concept of coronary insufficiency, which thus also includes patients with microvascular angina.2,3
The incidence of refractory angina is believed to be growing, due to the aging of the population, increasing complexity of coronary artery disease and the presence of multiple comorbidities, all of which limit therapeutic options.1 However, significant differences in definitions, the heterogeneity of the affected patients, and ongoing developments in treatment, mean that there are currently no precise estimates of the true prevalence of this condition. The available evidence comes mainly from registries of catheterization laboratories from the late 1990s and early 2000s, and points to an estimated prevalence of between 9.3% and 15% of patients referred for coronary angiography.4–9 On this basis the ESC Joint Study Group on the treatment of refractory angina estimate an incidence of 30-50 000 new cases annually in Europe.3
Annual mortality in patients with refractory angina in an angina clinic registry was less than 4%/year, 3.9% in the first year, and 17.5% at five years.10 In a French real-world registry including 4184 patients with stable coronary disease, five-year mortality was around 16%, ranging from less than 2% in patients aged under 65 years with no history of heart failure and preserved ejection fraction to more than 50% in those aged over 65 years, heart failure and ejection fraction <40%.11 In randomized studies on highly selected populations comparing therapeutic strategies in stable coronary disease, mortality was 7.9% at 4.6 years in COURAGE,12 11.4% vs. 13.9% at five years in the CABG group vs. the PCI group in SYNTAX,13 and 9% vs. 8.3% at five years in ISCHEMIA for the invasive vs. conservative approach, respectively.14
Comparisons between the prognosis of refractory angina and that of stable asymptomatic coronary disease are limited by differences in the demographic characteristics, clinical context and comorbidities of the study populations.7 The prognosis of refractory angina would be expected to fit within the spectrum of stable disease when other clinically relevant factors are taken into account.10 However, patients with refractory angina suffer from greater disability, have worse quality of life, and consume more health care resources, including hospitalizations, diagnostic exams and therapies.15
Refractory angina is thus an emerging problem that requires effective treatment to mitigate symptoms and improve quality of life for individual patients, and from a societal standpoint to optimize the associated use of resources and costs.
Phenotypes of patients with refractory anginaAngina is a clinical diagnosis; additional investigation aims to determine the presence of obstructive coronary artery disease or ischemia, neither of which will necessarily be present.1
Obstructive coronary disease is the best-known therapeutic target, although other mechanisms, such as microvascular dysfunction, vasospasm and neurogenic or psychogenic factors, may give rise to symptoms in the absence of obstructive disease or after revascularization.
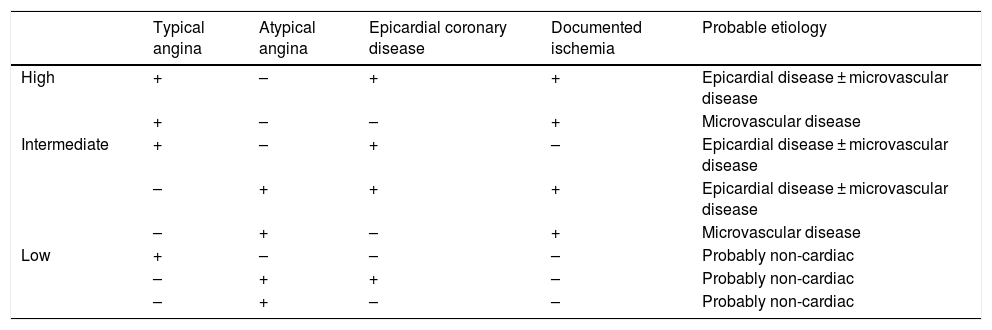
Medical therapy and revascularization currently relieve symptoms in around 80% of individuals with angina, but a varied group of patients have persistent symptoms.16 These patients should be classified as having chronic chest pain syndrome, which encompasses a range of entities from non-cardiac chest pain to refractory angina. Clinical (characteristics of symptoms), anatomical (presence of obstructive disease) and functional (presence of ischemia) data need to be brought together to determine the probability of refractory angina17 (Table 1).
Clinical probability of refractory angina.
| Typical angina | Atypical angina | Epicardial coronary disease | Documented ischemia | Probable etiology | |
|---|---|---|---|---|---|
| High | + | – | + | + | Epicardial disease ± microvascular disease |
| + | – | – | + | Microvascular disease | |
| Intermediate | + | – | + | – | Epicardial disease ± microvascular disease |
| – | + | + | + | Epicardial disease ± microvascular disease | |
| – | + | – | + | Microvascular disease | |
| Low | + | – | – | – | Probably non-cardiac |
| – | + | + | – | Probably non-cardiac | |
| – | + | – | – | Probably non-cardiac |
+: present; –: absent; ±: with or without.
Refractory angina is thus the clinical manifestation of a variety of entities including obstructive coronary disease, microvascular disease, vasospasm, myocardial disease and diastolic dysfunction.17,18
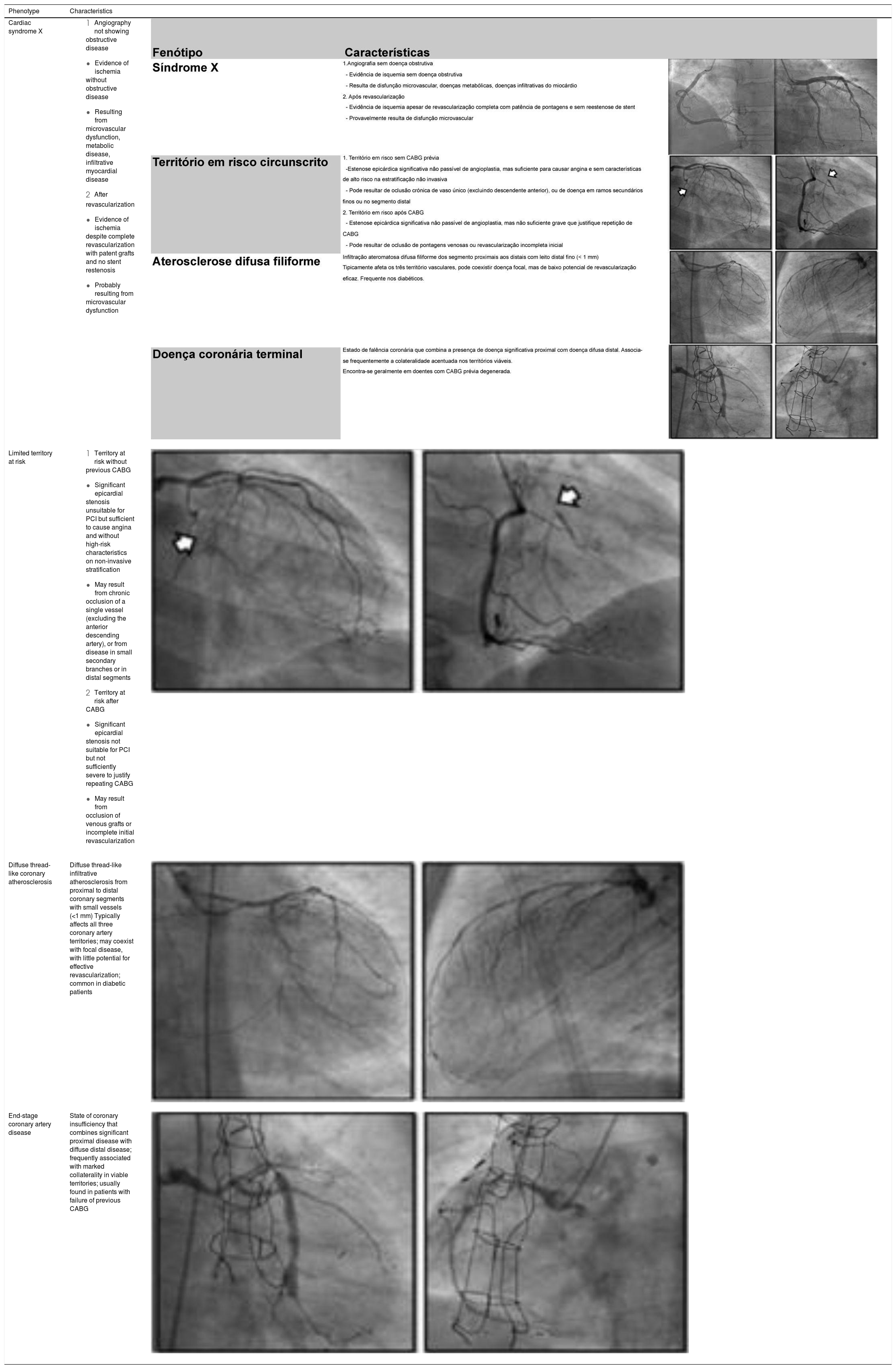
Four angiographic phenotypes of patients with refractory angina have been proposed2: (1) absence of obstructive coronary disease (suspected cardiac syndrome X); (2) limited territory at risk; (3) diffuse thread-like coronary atherosclerosis; and (4) end-stage coronary artery disease (Table 2).
Angiographic phenotypes of patients with refractory angina.
| Phenotype | Characteristics | |
|---|---|---|
| Cardiac syndrome X |
| |
| Limited territory at risk |
| |
| Diffuse thread-like coronary atherosclerosis | Diffuse thread-like infiltrative atherosclerosis from proximal to distal coronary segments with small vessels (<1 mm) Typically affects all three coronary artery territories; may coexist with focal disease, with little potential for effective revascularization; common in diabetic patients | |
| End-stage coronary artery disease | State of coronary insufficiency that combines significant proximal disease with diffuse distal disease; frequently associated with marked collaterality in viable territories; usually found in patients with failure of previous CABG |
CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention.
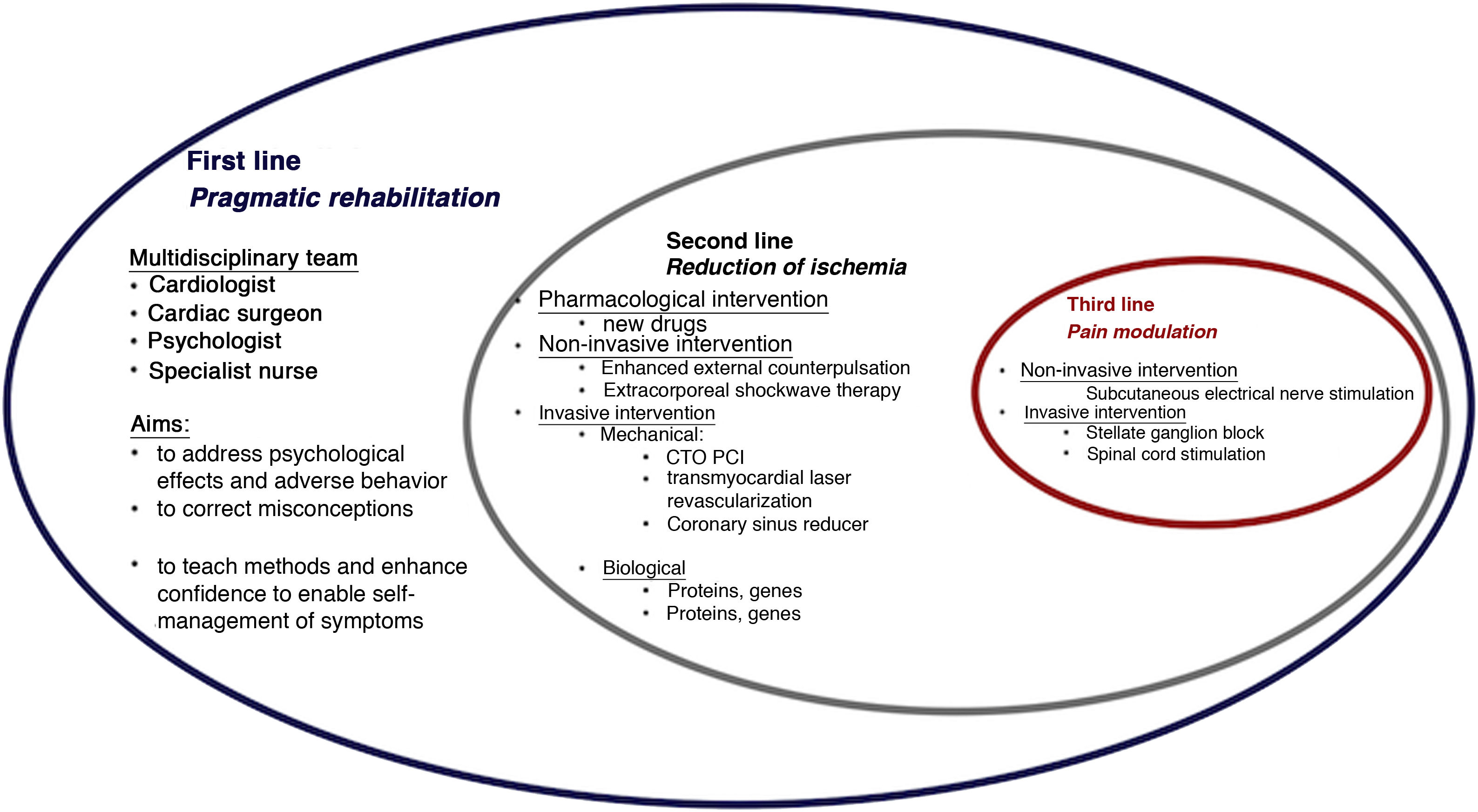
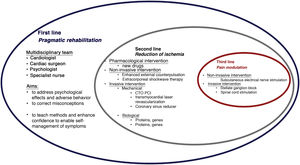
The paradigm of angina treatment is based on re-establishing the balance between oxygen supply and demand.19 However, in patients with refractory angina, the symptoms result from the interaction between multiple mechanisms, and so the approach must be holistic (Figure 1).
The first-line intervention in all cases should be pragmatic rehabilitation, in order to correct patients’ misconceptions, enhance their self-confidence and teach them techniques for self-management of symptoms. Subsequent interventions should be personalized according to the main mechanism causing symptoms and to local resources.
The second-line intervention is reduction of ischemia, for which both non-invasive and invasive techniques have been developed. The former include enhanced external counterpulsation20 and extracorporeal shockwave therapy,21 while invasive techniques include mechanical interventions such as PCI of chronic total occlusions (CTOs),22,23 transmyocardial laser revascularization24,25 and coronary sinus reduction,26–32 and biological interventions such as gene and cell therapy.33,34
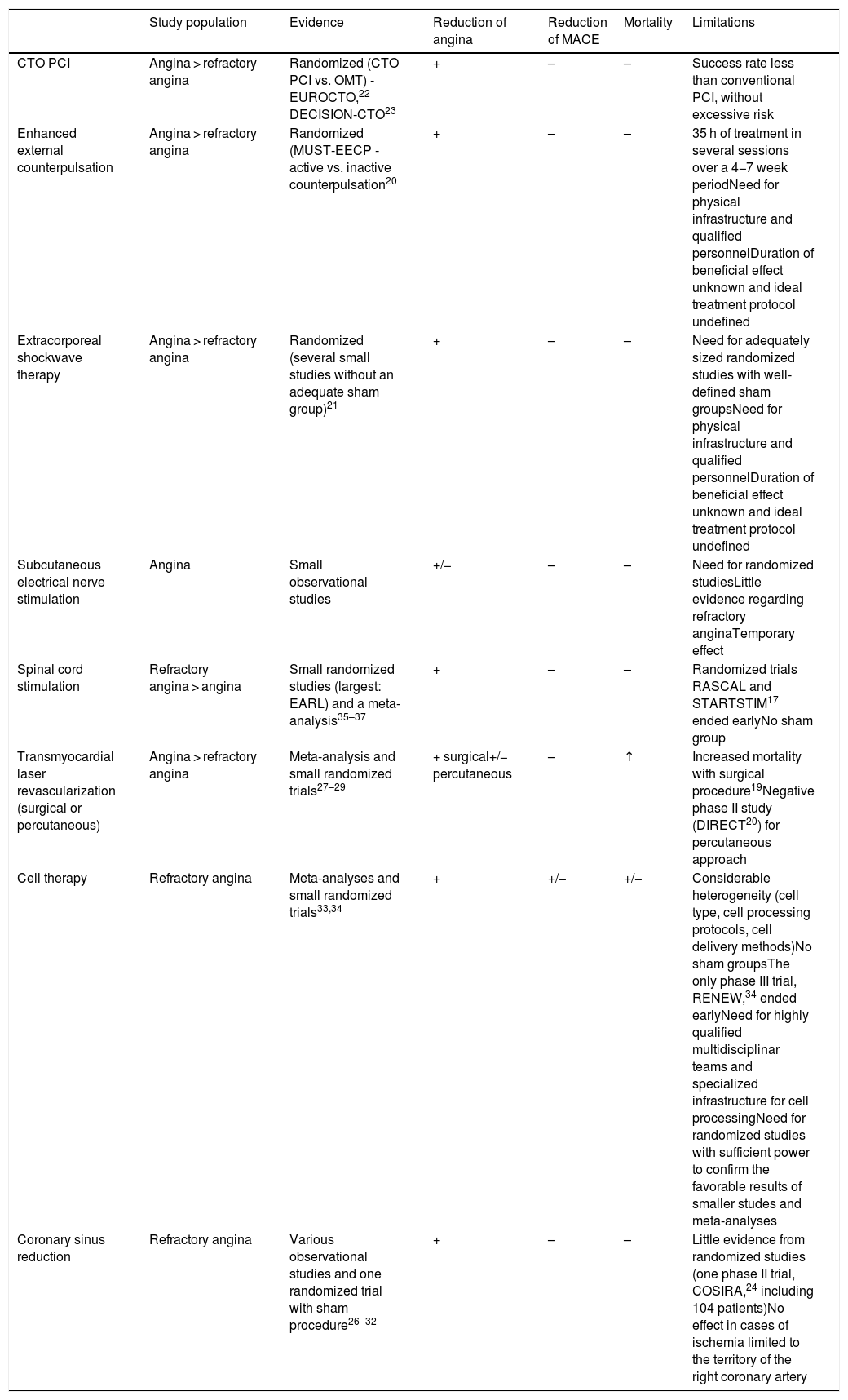
Third-line intervention encompasses pain modulation using non-invasive techniques such as subcutaneous electrical nerve stimulation and invasive methods such as stellate ganglion block and spinal cord stimulation.17,35–37Table 3 summarizes the main characteristics, results and limitations of studies on non-pharmacological angina therapy. Overall the evidence suggests that only cell therapy, CTO PCI and coronary sinus reduction are effective and safe for reducing angina.
Non-pharmacological angina therapy.
| Study population | Evidence | Reduction of angina | Reduction of MACE | Mortality | Limitations | |
|---|---|---|---|---|---|---|
| CTO PCI | Angina > refractory angina | Randomized (CTO PCI vs. OMT) - EUROCTO,22 DECISION-CTO23 | + | – | – | Success rate less than conventional PCI, without excessive risk |
| Enhanced external counterpulsation | Angina > refractory angina | Randomized (MUST-EECP - active vs. inactive counterpulsation20 | + | – | – | 35 h of treatment in several sessions over a 4−7 week periodNeed for physical infrastructure and qualified personnelDuration of beneficial effect unknown and ideal treatment protocol undefined |
| Extracorporeal shockwave therapy | Angina > refractory angina | Randomized (several small studies without an adequate sham group)21 | + | – | – | Need for adequately sized randomized studies with well-defined sham groupsNeed for physical infrastructure and qualified personnelDuration of beneficial effect unknown and ideal treatment protocol undefined |
| Subcutaneous electrical nerve stimulation | Angina | Small observational studies | +/− | – | – | Need for randomized studiesLittle evidence regarding refractory anginaTemporary effect |
| Spinal cord stimulation | Refractory angina > angina | Small randomized studies (largest: EARL) and a meta-analysis35–37 | + | – | – | Randomized trials RASCAL and STARTSTIM17 ended earlyNo sham group |
| Transmyocardial laser revascularization (surgical or percutaneous) | Angina > refractory angina | Meta-analysis and small randomized trials27–29 | + surgical+/− percutaneous | – | ↑ | Increased mortality with surgical procedure19Negative phase II study (DIRECT20) for percutaneous approach |
| Cell therapy | Refractory angina | Meta-analyses and small randomized trials33,34 | + | +/− | +/− | Considerable heterogeneity (cell type, cell processing protocols, cell delivery methods)No sham groupsThe only phase III trial, RENEW,34 ended earlyNeed for highly qualified multidisciplinar teams and specialized infrastructure for cell processingNeed for randomized studies with sufficient power to confirm the favorable results of smaller studes and meta-analyses |
| Coronary sinus reduction | Refractory angina | Various observational studies and one randomized trial with sham procedure26–32 | + | – | – | Little evidence from randomized studies (one phase II trial, COSIRA,24 including 104 patients)No effect in cases of ischemia limited to the territory of the right coronary artery |
CTO PCI: percutaneous coronary intervention for chronic total occlusion; COSIRA: Coronary Sinus Reducer for Treatment of Refractory Angina; DECISION-CTO: Drug-Eluting Stent Implantation versus Optimal Medical Treatment in Patients with Chronic Total Occlusion; DIRECT: Direct Myocardial Revascularization in Regeneration of Endomyocardial Channels Trial; EUROCTO: European Study on the Utilization of Revascularization vs. Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions; MACE: major adverse cardiovascular event; OMT: optimal medical therapy; RASCAL: Effectiveness and Cost-Effectiveness of Spinal Cord Stimulation for Refractory Angina; RENEW: Efficacy and Safety of Intramyocardial Autologous CD34+ Cell Administration in Patients With Refractory Angina; STARTSTIM: Stimulation Therapy for Angina RefracTory to Standard Treatments, Interventions, and Medications.
In the 1950s, in the pre-coronary angiography era38 when treatment for angina was limited to empirical symptomatic relief with nitrates, atropine, phenobarbital or narcotics,39 Claude Beck was engaged in investigating various surgical interventions to treat angina, basically of two types: those aiming at indirect revascularization (aiming to promote neoangiogenesis and collaterality through mechanical insults to the pericardium and myocardium), and those intended to reduce the diameter of the coronary sinus.40 Of these efforts, only ligation of the coronary sinus to reduce its lumen by 60-70%, to around 3 mm, proved to be clinically useful. A comparative study including 185 patients showed lower five-year mortality (13% in the treatment group vs. 30% in untreated patients) and angina relief, with 90% of patients reporting complete or significant symptomatic relief.41 However, interest in this therapy waned as further developments led to successes in both medical therapy and direct surgical revascularization to treat stable coronary disease.
Interest in intermittent percutaneous reduction or occlusion of the coronary sinus revived in the 1980s, in the context of acute coronary syndrome42 and coronary artery bypass grafting.43 The technique was further investigated and refined in the 2000s, but despite the benefits demonstrated in reducing infarct size and in recovery of myocardial function,44 it never became established in the clinical arena.
More recently, as a result of the growing numbers of patients with refractory angina, the suboptimal performance of current alternatives, and the positive results from pioneering studies, interest in this potential therapeutic target has reawakened. An implantable device for percutaneous intraluminal delivery designed to achieve persistent coronary sinus reduction, similarly to Beck’s surgical external ligation, has been developed and studied.
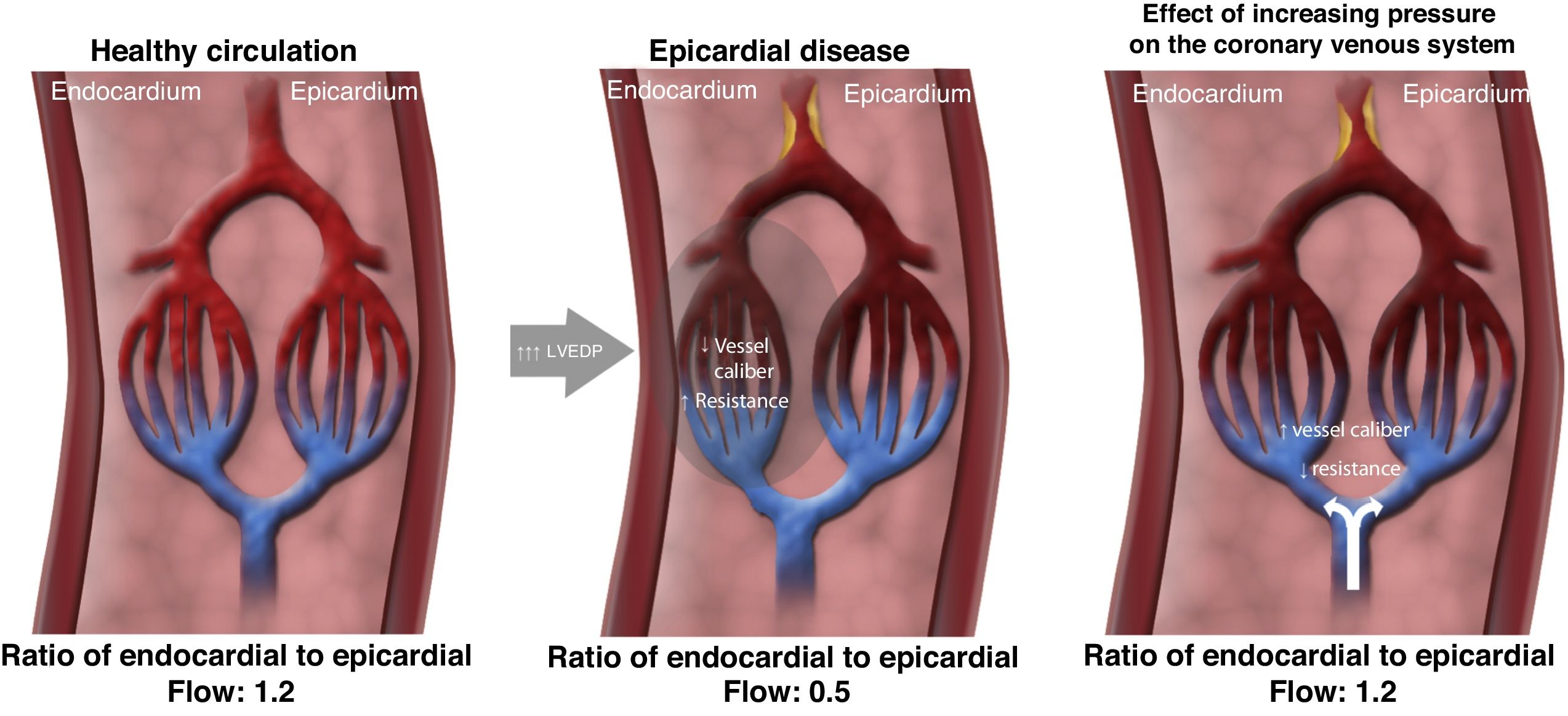
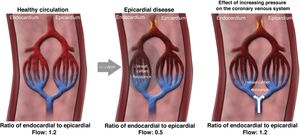
Possible mechanisms of actionIn a healthy heart, exercise leads to sympathetic-mediated vasoconstriction of the subepicardial vessels that directs more flow to the subendocardial layer.45–47 In the presence of obstructive epicardial coronary disease or microvascular disease, the regulatory mechanisms are compromised: the distribution of perfusion no longer favors the subendocardial layer, leading to ischemia of this region, reducing contractility, which in turn increases left ventricular filling pressures and further imbalance in the ratio between subendocardial and subepicardial perfusion. The consequence is anginal symptoms and exertional dyspnea.45,46
Although the mechanism of action of coronary sinus occlusion has not been definitively identified, it is thought that elevated pressure at the venous end of the coronary circulation could lead to a slight dilatation of the arterioles, thereby increasing the cross-sectional area of the vascular bed and thus significantly reducing the resistance in the subendocardial layer. The resulting redistribution of flow from subepicardial to ischemic subendocardial regions corrects the ratio between subepicardial and subendocardial perfusion, which in turn leads to symptom relief (Figure 2).48
Mechanism of action of coronary sinus reduction (from Ref. 46). LVEDP: left ventricular end-diastolic pressure.
Other authors have suggested that increased pressure in the coronary venous system promotes neoangiogenesis, which is responsible for the improvement in subendocardial perfusion and symptoms 49
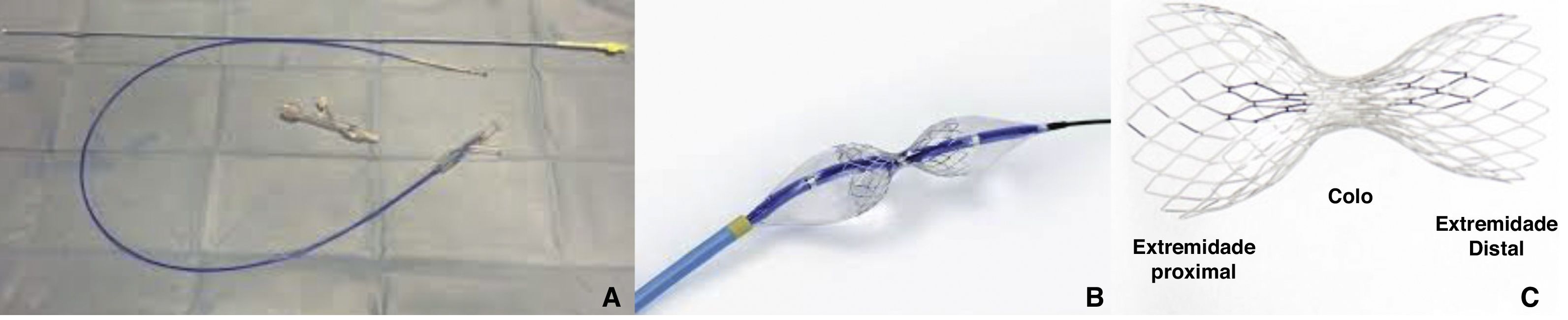
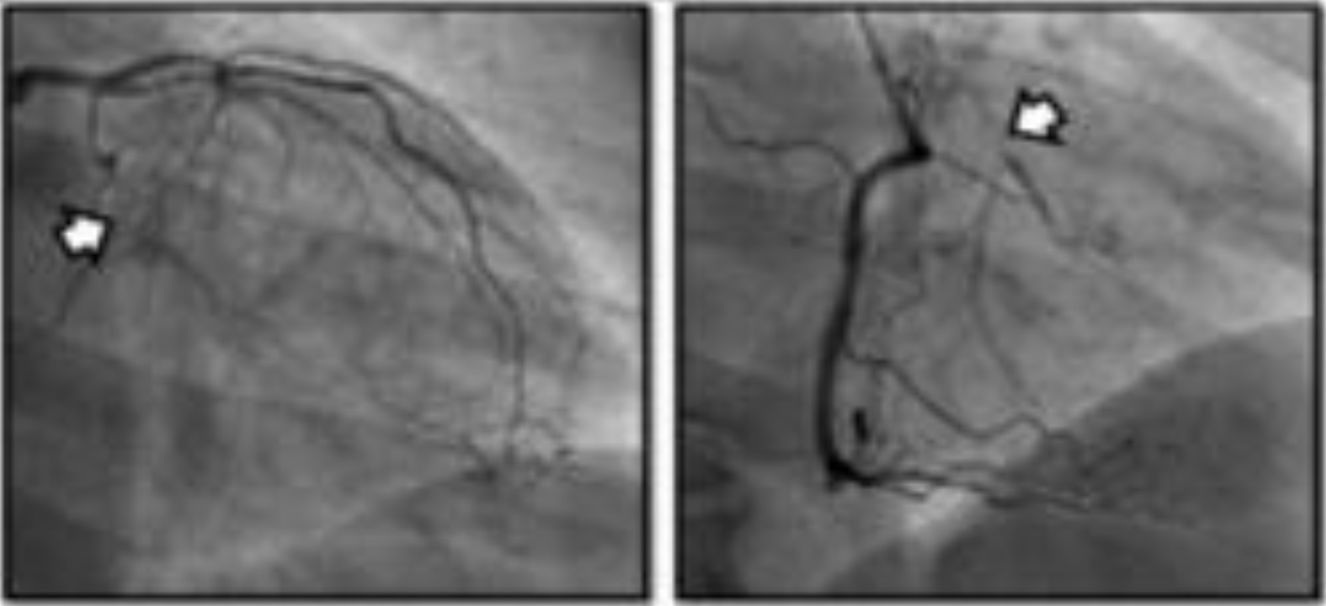
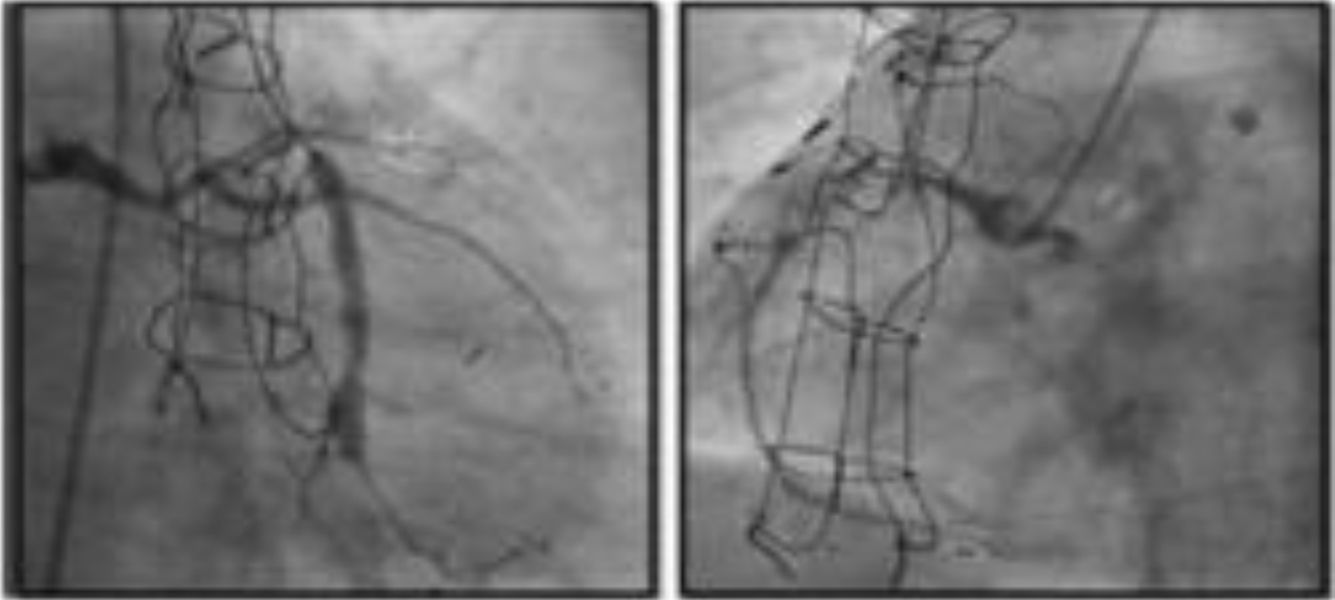
DeviceThe only coronary sinus reducer device currently available and approved for clinical use is the Neovasc Reducer™ (Neovasc Inc., Richmond, BC, Canada). The device consists of a stainless steel mesh mounted in an hourglass-shaped balloon, which after inflation adapts to the tapering conformation of the coronary sinus with two asymmetrical extremities (the proximal larger than the distal) and a narrow central portion that following endothelialization (which takes 4–6 weeks) creates a pressure gradient between the venous circulation corresponding to the territory of the left coronary artery and the right atrium. The semicompliant balloon is available in only one size and the device’s final expanded diameters (9−14 mm) are dependent on the filling pressure, enabling it to adapt to fit the range of anatomies encountered in most patients, while the diameter of the central neck will always be 3 mm (Figure 3).
The device is implanted with a 10-20% oversizing -20 at each end relative to the coronary sinus diameter, in order to anchor the device and to trigger the vascular injury that will induce tissue regrowth and endothelialization.
After implantation, the device can be balloon expanded at any time, so that further interventions can be performed through the coronary sinus if required.
EvidenceSince the coronary sinus Reducer was first used in humans 15 years ago, more than 1000 devices have been implanted worldwide. No long-term complications have been reported.
The device’s performance was first tested in animal models and then progressed to clinical assessment in small proof-of-concept studies, registries and one randomized trial. All studies on humans have included only patients with refractory angina under optimal medical therapy who were not candidates for revascularization.
Preclinical studiesThe device’s feasibility, efficacy and safety were first tested in a study using porcine models.50 Feasibility and safety were assessed in one arm of the study and efficacy in the other. Overall implantation success was 100% in 32 animals, with no immediate procedure-related complications. In the safety arm (n=26), histological study of devices retrieved between the first and sixth months after implantation presented favorable tissue repair, with the central portion demonstrating progressive endothelialization in continuity with the remainder of the vessel lumen. No device-related complications such as migration, thrombosis, stenosis, occlusion or erosion were observed when the animals were sacrificed. In the efficacy arm, 22 animals underwent artificial constriction of the proximal circumflex artery; of these, eight presented wall motion abnormalities in this territory on pharmacological stress echocardiography with dobutamine and perfusion alterations on contrast echocardiography. The Reducer device was then implanted in four of these eight animals, in whom a 50% reduction in ischemia was seen at six months, compared to absence of improvement in the four untreated animals. In the untreated group, three out of four animals died prematurely, compared to no mortality in the treatment group.
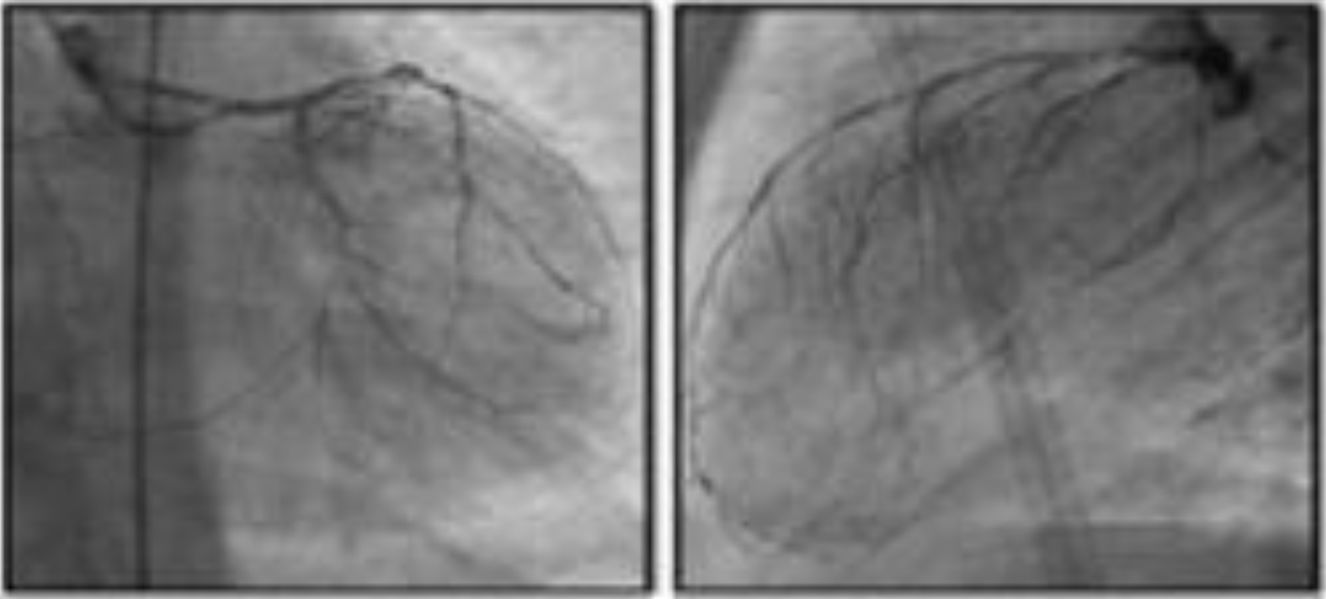
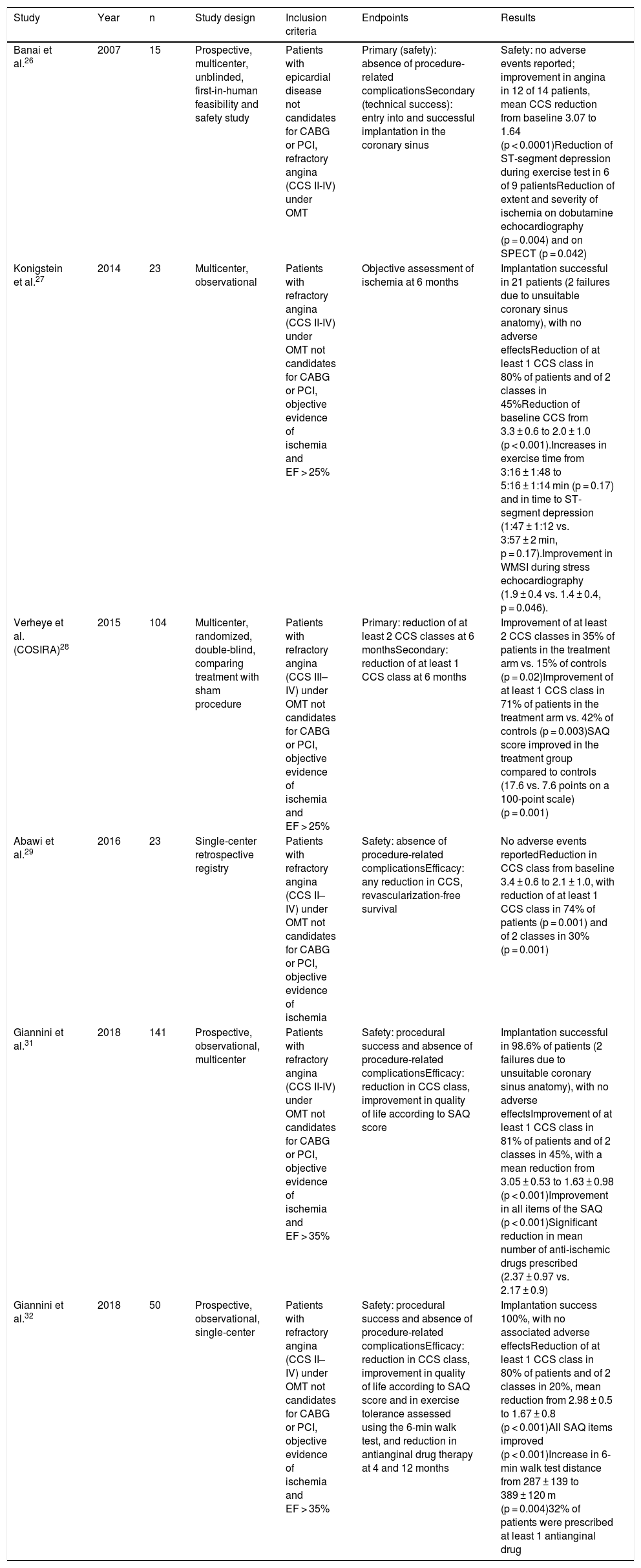
Clinical studiesThe first feasibility and safety study in humans enrolled 15 patients with refractory angina and evidence of ischemia on single-photon emission computed tomography (SPECT) or stress echocardiography.26 Implantation was successful in all subjects. At six months all devices were patent on computed tomography angiography, Canadian Cardiovascular Society (CCS) angina class had improved in 12 patients from a mean of 3 prior to implantation to 1.6, and reductions in extent and severity of ischemia were documented by SPECT and stress echocardiography. Device patency and location were reassessed by computed tomography angiography at three years and no cases of occlusion or migration were found51 (Table 4).
Clinical studies assessing the coronary sinus Reducer device in patients with refractory angina.
| Study | Year | n | Study design | Inclusion criteria | Endpoints | Results |
|---|---|---|---|---|---|---|
| Banai et al.26 | 2007 | 15 | Prospective, multicenter, unblinded, first-in-human feasibility and safety study | Patients with epicardial disease not candidates for CABG or PCI, refractory angina (CCS II-IV) under OMT | Primary (safety): absence of procedure-related complicationsSecondary (technical success): entry into and successful implantation in the coronary sinus | Safety: no adverse events reported; improvement in angina in 12 of 14 patients, mean CCS reduction from baseline 3.07 to 1.64 (p < 0.0001)Reduction of ST-segment depression during exercise test in 6 of 9 patientsReduction of extent and severity of ischemia on dobutamine echocardiography (p = 0.004) and on SPECT (p = 0.042) |
| Konigstein et al.27 | 2014 | 23 | Multicenter, observational | Patients with refractory angina (CCS II-IV) under OMT not candidates for CABG or PCI, objective evidence of ischemia and EF > 25% | Objective assessment of ischemia at 6 months | Implantation successful in 21 patients (2 failures due to unsuitable coronary sinus anatomy), with no adverse effectsReduction of at least 1 CCS class in 80% of patients and of 2 classes in 45%Reduction of baseline CCS from 3.3 ± 0.6 to 2.0 ± 1.0 (p < 0.001).Increases in exercise time from 3:16 ± 1:48 to 5:16 ± 1:14 min (p = 0.17) and in time to ST-segment depression (1:47 ± 1:12 vs. 3:57 ± 2 min, p = 0.17).Improvement in WMSI during stress echocardiography (1.9 ± 0.4 vs. 1.4 ± 0.4, p = 0.046). |
| Verheye et al.(COSIRA)28 | 2015 | 104 | Multicenter, randomized, double-blind, comparing treatment with sham procedure | Patients with refractory angina (CCS III–IV) under OMT not candidates for CABG or PCI, objective evidence of ischemia and EF > 25% | Primary: reduction of at least 2 CCS classes at 6 monthsSecondary: reduction of at least 1 CCS class at 6 months | Improvement of at least 2 CCS classes in 35% of patients in the treatment arm vs. 15% of controls (p = 0.02)Improvement of at least 1 CCS class in 71% of patients in the treatment arm vs. 42% of controls (p = 0.003)SAQ score improved in the treatment group compared to controls (17.6 vs. 7.6 points on a 100-point scale) (p = 0.001) |
| Abawi et al.29 | 2016 | 23 | Single-center retrospective registry | Patients with refractory angina (CCS II–IV) under OMT not candidates for CABG or PCI, objective evidence of ischemia | Safety: absence of procedure-related complicationsEfficacy: any reduction in CCS, revascularization-free survival | No adverse events reportedReduction in CCS class from baseline 3.4 ± 0.6 to 2.1 ± 1.0, with reduction of at least 1 CCS class in 74% of patients (p = 0.001) and of 2 classes in 30% (p = 0.001) |
| Giannini et al.31 | 2018 | 141 | Prospective, observational, multicenter | Patients with refractory angina (CCS II-IV) under OMT not candidates for CABG or PCI, objective evidence of ischemia and EF > 35% | Safety: procedural success and absence of procedure-related complicationsEfficacy: reduction in CCS class, improvement in quality of life according to SAQ score | Implantation successful in 98.6% of patients (2 failures due to unsuitable coronary sinus anatomy), with no adverse effectsImprovement of at least 1 CCS class in 81% of patients and of 2 classes in 45%, with a mean reduction from 3.05 ± 0.53 to 1.63 ± 0.98 (p < 0.001)Improvement in all items of the SAQ (p < 0.001)Significant reduction in mean number of anti-ischemic drugs prescribed (2.37 ± 0.97 vs. 2.17 ± 0.9) |
| Giannini et al.32 | 2018 | 50 | Prospective, observational, single-center | Patients with refractory angina (CCS II–IV) under OMT not candidates for CABG or PCI, objective evidence of ischemia and EF > 35% | Safety: procedural success and absence of procedure-related complicationsEfficacy: reduction in CCS class, improvement in quality of life according to SAQ score and in exercise tolerance assessed using the 6-min walk test, and reduction in antianginal drug therapy at 4 and 12 months | Implantation success 100%, with no associated adverse effectsReduction of at least 1 CCS class in 80% of patients and of 2 classes in 20%, mean reduction from 2.98 ± 0.5 to 1.67 ± 0.8 (p < 0.001)All SAQ items improved (p < 0.001)Increase in 6-min walk test distance from 287 ± 139 to 389 ± 120 m (p = 0.004)32% of patients were prescribed at least 1 antianginal drug |
CABG: coronary artery bypass grafting; CCS: Canadian Cardiovascular Society angina class; EF: ejection fraction; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; SAQ: Seattle Angina Questionnaire quality of life score; SPECT: single-photon emission computed tomography; WMSI: wall motion score index.
To separate the device’s mechanistic antianginal effect from the placebo effect frequently attributed to angina therapies,52,53 the same investigators designed a randomized, double-blind, multicenter study, the COSIRA trial, in which the control group underwent a sham procedure.28 The study analyzed 104 patients with refractory angina (CCS III–IV) and evidence of ischemia over a period of 38 months, the small sample size reflecting the low prevalence of refractory angina among the population with stable coronary disease as well as the restrictive inclusion criteria used. Improvement of at least one CCS class was observed in 42% of the control group and in 71% of those who received the device (p = 0.003) and the primary endpoint (improvement of two or more CCS angina classes) was reached in 35% of the treatment group as opposed to 15% of the control group (p = 0.024). Quality of life as assessed with the Seattle Angina Questionnaire (SAQ) was significantly improved in the treatment group as compared with the control group (17.6 vs. 7.6 points, p = 0.03). There were no significant differences between groups in improvement in exercise time or in the extent of ischemia as measured by the wall-motion index (WMI). Even so, mean exercise time improved by 59 s (13%) in the treatment group and by only 4 s (1%) in the control group (p = 0.07), which from a clinical standpoint may represent asignificant benefit. In the analysis of extent and severity of ischemia, in which only the 11 segments attributed to the left coronary artery territory were assessed (modified wall motion index), an improvement of 13% was seen in the treatment group compared to 3% in the control group, which although not statistically significant, showed a clear tendency for ischemia reduction. There were no significant differences between the groups in adverse events, either procedure-related or in the first year of follow-up.
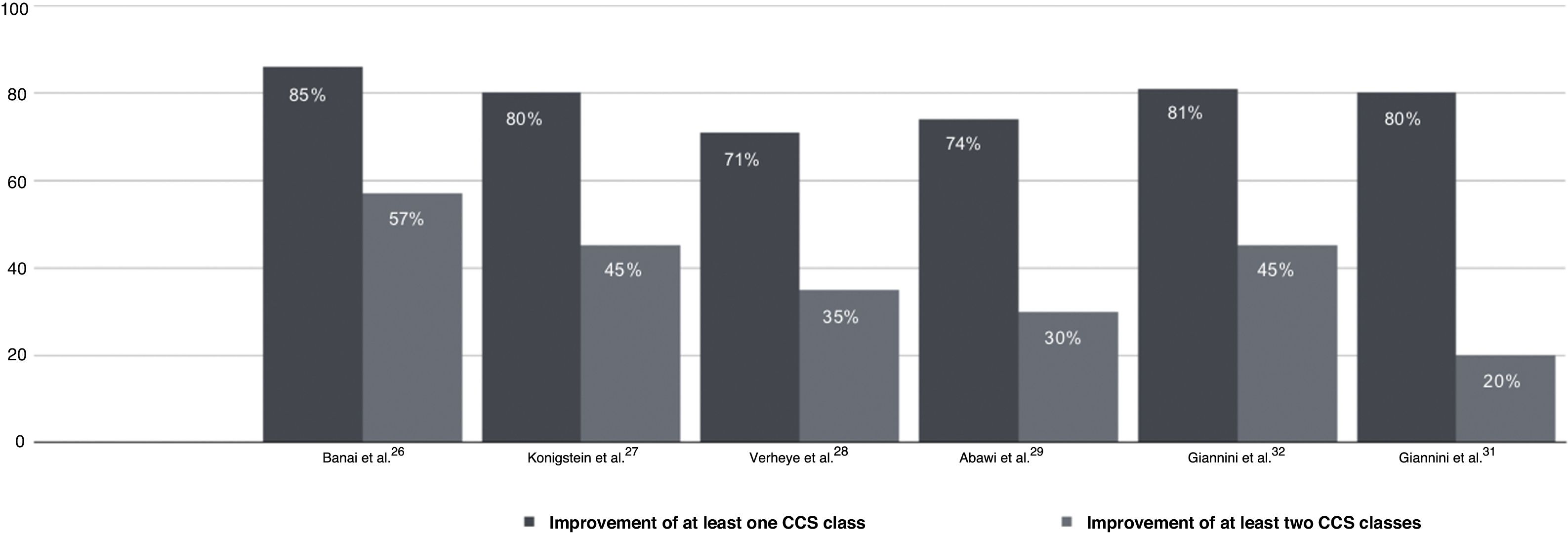
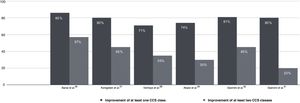
Multiple post-marketing observational studies and registries have consistently demonstrated improvements of at least one CCS class in 70–80% of patients, with the proportion of non-responders remaining at steady around 20–30% (Figure 4).29,31,32,54
Although most patients included in these studies and registries presented obstructive coronary disease and patients with microvascular disease were under-represented, no difference was noted in the therapeutic effect of the Reducer device between these two phenotypes of refractory angina. A small study in two Italian centers, selected (from a pool of patients referred for coronary angiography) eight patients with refractory angina (CCS III or IV), non-invasive evidence of myocardial ischemia and no obstructive disease, for compassionate Reducer implantation. Median CCS class decreased from 3.0 to 1.5 (p = 0.014), SAQ scores improved, 40% of patients discontinued at least one antianginal agent, and 6-min walk test distance improved. In all three patients who underwent cardiac magnetic resonance before and after Reducer implantation, myocardial perfusion reserve index improved.55 These results suggest that the benefits of coronary sinus reduction potentially extend to patients with microvascular disease.
Although the improvements in symptoms and quality of life seen in observational studies are in line with the results of the treatment arm in the COSIRA trial, when interpreting these results it is essential to keep in mind the strength of the placebo effect in patients undergoing this intervention. Randomized double-blind sham-controlled trials suitably powered to identify differences in objective measures of ischemia will clarify to what extent symptomatic improvement correlates with reductions in ischemia.
On the basis of the evidence presented, which although consistent is still scarce (only one small randomized trial and non-randomized evidence limited by the known strengthing of the placebo effect associated with angina therapy 24,53), the 2019 ESC guidelines for the first time recommended consideration of the use of the coronary sinus Reducer device (class IIb) among the treatment options for refractory angina.1
In order to strengthen the available evidence on the potential therapeutic benefit of the Reducer, a second randomized trial is planned (COSIRA II), with a similar design to COSIRA I, aiming to include 380 patients in the US and Canada.
Treatment with a coronary sinus Reducer thus appears to be safe and potentially effective, and can be implemented in any catheterization laboratory.
Non-respondersFor reasons that are not fully understood, 20–25% of patients receiving the Reducer do not present symptomatic improvement. This may be related to variations in the cardiac venous circulation, as venous drainage systems other than the coronary sinus could diminish the benefit of coronary sinus reduction. Other factors potentially associated with failure to respond are ischemia in the right coronary artery territory, concomitant heart disease that raises right atrial pressure by >15 mmHg, very high left ventricular filling pressure in patients with heart failure, incomplete endothelialization of the metal mesh resulting in an insufficient gradient, and the possibility that the patient’s pain may not be anginal.56,57
Cost-effectivenessA retrospective multicenter cohort study including 215 patients with refractory angina who underwent implantation of the Reducer device in Belgium, The Netherlands and Italy assessed the associated costs to the health system of each country.58 The authors compared the pre-implantation period (standard of care) with the post-implantation period (one-year follow-up) and observed a significant reduction in angina-driven hospitalizations, outpatient visits, coronary angiograms, and PCI procedures per patient-year. This translated into incremental cost-effectiveness ratios (ICERs) of 53 197, 34 948, and 63 146 euros per quality-adjusted life year (QALY) gained in Belgium, the Netherlands, and Italy, respectively, which is below the cost-effectiveness threshold set by the World Health Organization (WHO)59 (ICER less than three times per capita gross domestic product) in 99.9%, 100% and 93.3% of patients, respectively, in the three countries.
Interpretation of this study should take into consideration that it had no formal control group, the follow-up period was short and neither the costs of drug treatment nor indirect costs were included. However, bearing in mind the chronic nature of the symptoms of refractory angina, the progressive natural history of coronary disease and the clinical benefits derived from use of the Reducer device, the results suggest that in these countries there were economic benefits. The cost-effectiveness threshold for Portugal according to the latest figures for per capita gross domestic product 60 as defined by the WHO is 65 040 euros/QALY, which is higher than the ICERs calculated for Belgium, the Netherlands, and Italy, and it is thus to be expected that in the Portuguese setting the Reducer device would also be cost-effective..
ConclusionThe coronary sinus Reducer device is a promising therapeutic alternative for relieving symptoms and improving quality of life in selected patients with refractory angina. It still lacks additional validation in suitably designed and sized clinical trials.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Madeira S, Brízido C, Raposo L, Brito J, Vale N, Leal S, et al., Tratamento não farmacológico da angina refratária. Dispositivo de redução do seio coronário, uma nova alternativa terapêutica. Rev Port Cardiol. 2021;40:371–382.