Hypertrophic cardiomyopathy is an inherited cardiac disease and a major cause of heart failure and sudden death. Although it was first described more than 50 years ago, sarcomeric hypertrophic cardiomyopathy still lacks a disease-specific treatment: the drugs routinely used alleviate symptoms but do not prevent or revert the phenotype. With recent advances in knowledge of the genetics and pathophysiology of hypertrophic cardiomyopathy, new genetic and pharmacological approaches have recently been identified and studied that, by influencing different pathways involved in this disease, have the potential to function as disease-modifying therapies. These promising new pharmacological and genetic therapies will be the focus of this review.
A miocardiopatia hipertrófica é uma doença cardíaca hereditária e uma causa major de insuficiência cardíaca e morte súbita. Apesar de descrita há mais de 50 anos, a miocardiopatia hipertrófica sarcomérica ainda não apresenta uma terapêutica farmacológica especifica de doença: os fármacos utilizados por rotina oferecem alívio sintomático mas não previnem ou revertem o fenótipo característico. Os avanços no conhecimento relativo à genética e fisiopatologia da miocardiopatia hipertrófica permitiram, recentemente, descobrir e estudar novas abordagens genéticas e farmacológicas que, ao influenciar diferentes vias envolvidas nesta doença, têm o potencial de funcionar como terapêuticas modificadoras. Estas novas e promissoras terapêuticas são o foco desta revisão.
Hypertrophic cardiomyopathy (HCM) is a disease of the myocardium and a major cause of sudden death and heart failure.1–8 It is characterized by the presence of left ventricular hypertrophy (LVH) unexplained by loading conditions sufficient to cause the observed abnormality. HCM is usually detected by echocardiography or cardiac magnetic resonance (CMR).1–8 Its prevalence, based on echocardiographic data, is estimated at 1/500 individuals.9 However, the prevalence of carriers of genetic variants known to cause HCM is around 1/200 individuals.9
HCM is usually an autosomal dominant genetic disorder.1–8 More than 1500 HCM-related mutations have been identified in over 11 genes, the majority of which affect genes encoding sarcomere proteins. The most common are the genes for beta-myosin heavy chain (MYH7) and for cardiac myosin-binding protein C (MYBPC3).1–6
Histologically, HCM is characterized by disorganized myocardial architecture with cardiomyocyte and myofibril disarray, intramural coronary artery wall thickening and interstitial fibrosis.1–6 Hypertrophy usually develops during growth spurts, especially in puberty and adolescence; it is normally present by the beginning of adulthood.2,5,10 Some mutations can, however, lead to late-onset hypertrophic expression of the disease.11
Clinical presentation is heterogeneous, and onset can occur at any stage of life.1,3,5–7 Most individuals with HCM live to an advanced age with few or no symptoms.1,3,5–7 However, affected individuals can develop symptoms and complications, such as heart failure, which affects approximately 50% of patients; atrial fibrillation, which is present in 25% of patients and leads to an increased risk of embolic events; and even sudden death, which although its annual incidence is less than 1%, may be the first and only disease manifestation.1,3,5–7,9 Overall, in spite of its high morbidity, sarcomeric HCM is nowadays a relatively benign disease, with an annual cardiovascular mortality of 1-2%.1
Therapy currently focuses on alleviating symptoms and preventing disease complications.1,3,5–8 Treatment options include placement of an implantable cardioverter-defibrillator to prevent sudden death, pharmacological therapy (or radiofrequency ablation) to control and treat atrial fibrillation in order to prevent embolic events, and pharmacological or invasive treatment (surgical myectomy or alcohol septal ablation) for relief of left ventricular outflow tract (LVOT) obstruction in refractory symptomatic cases.1,3,5–8 It should be noted that pharmacological therapy for the symptoms of heart failure depends on whether the patient has obstructive or non-obstructive HCM. In obstructive HCM or non-obstructive HCM with ejection fraction >50%, beta-blockers, calcium channel blockers, disopyramide (obstructive HCM) and diuretics are the therapy of choice, while patients with non-obstructive HCM and reduced ejection fraction should be treated with beta-blockers and angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and possibly diuretics and mineralocorticoid receptor antagonists (MRAs).1 Patients with progressive cardiac dysfunction may also be candidates for heart transplantation.1,3,5–8
Although HCM was first described more than 50 years ago, evidence-based therapies are scarce and there have been few clinical trials, with small numbers of patients, evaluating the efficacy of pharmacological treatments for this disease.7,8,12–14 In recent decades, advances in the understanding of HCM have led to the discovery of new approaches which may influence its complex pathophysiology, alter its natural history and act as disease-modifying therapies.7,8,12–14 Carriers of HCM-associated mutations which are not yet expressed phenotypically (genotype-positive, phenotype-negative individuals) are a population of particular interest in whom development of the disease phenotype may be prevented.
The purpose of this review is to discuss the most recent data and developments in new therapeutic approaches to HCM.
New therapeutic perspectives in hypertrophic cardiomyopathyDiltiazemDiltiazem is a non-dihydropyridine L-type calcium channel blocker indicated as a second-line therapy for symptomatic relief in HCM, since it is less effective than beta-blockers.1,3,5–8 It is also used to control ventricular rate in patients with atrial fibrillation.1,3,5–8
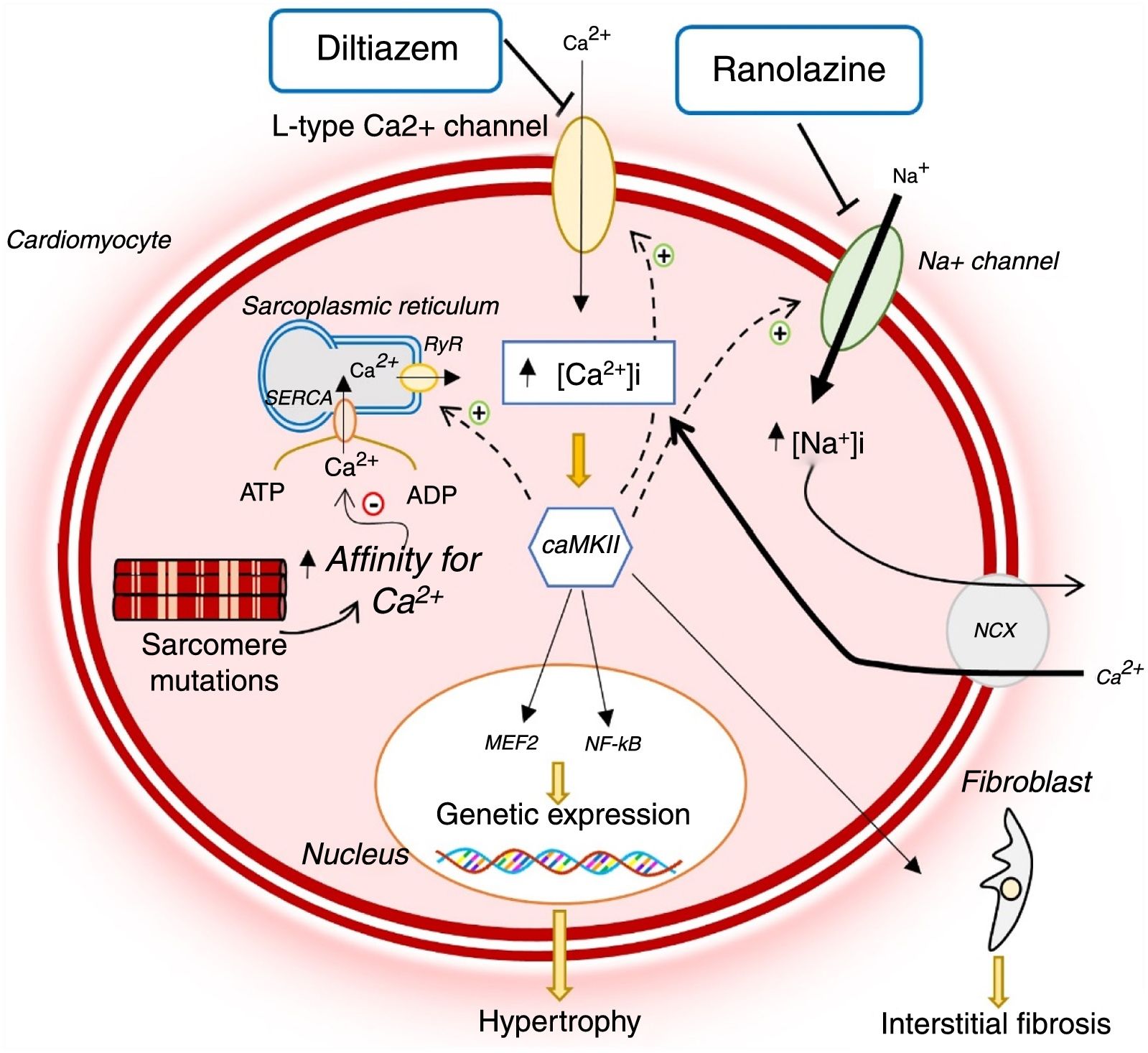
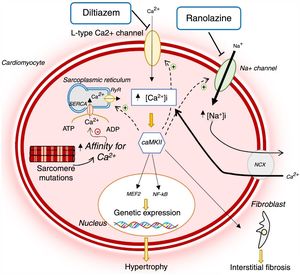
HCM-associated sarcomere mutations are now known to lead to early dysregulation of intracellular Ca2+ homeostasis (Figure 1).12,14–16 These mutations increase sarcomeric myofilament sensitivity and affinity for Ca2+, thus preventing its reuptake to the sarcoplasmic reticulum and efflux from the cardiomyocyte during diastole.12,14–18 In addition, sarcomere mutations result in increased energy expenditure in the cardiomyocyte, reducing the adenosine triphosphate (ATP) available for the ion pumps that regulate intracellular Ca2+ levels (Figure 2).12,14–16 This dysregulation leads to intracellular accumulation of Ca2+, which in addition to preventing cell relaxation, causes diastolic dysfunction and increased risk of arrhythmias, and activates signaling pathways which contribute to the adverse myocardial remodeling that is characteristic of HCM (Figure 1).12,14–16
Changes in Ca2+ homeostasis and in late sodium current in hypertrophic cardiomyopathy and mechanism of action of diltiazem and ranolazine.12,14,21 AMP: adenosine monophosphate; ATP: adenosine triphosphate; CaMKII: Ca2+/calmodulin-dependent protein kinase II; INaL: late sodium current; MEF2: myocyte enhancer factor 2; NCX: sodium/calcium exchanger; NF-κB: nuclear factor kappa B; RyR: ryanodine receptor; SERCA: sarco/endoplasmic reticulum Ca2+-ATPase.
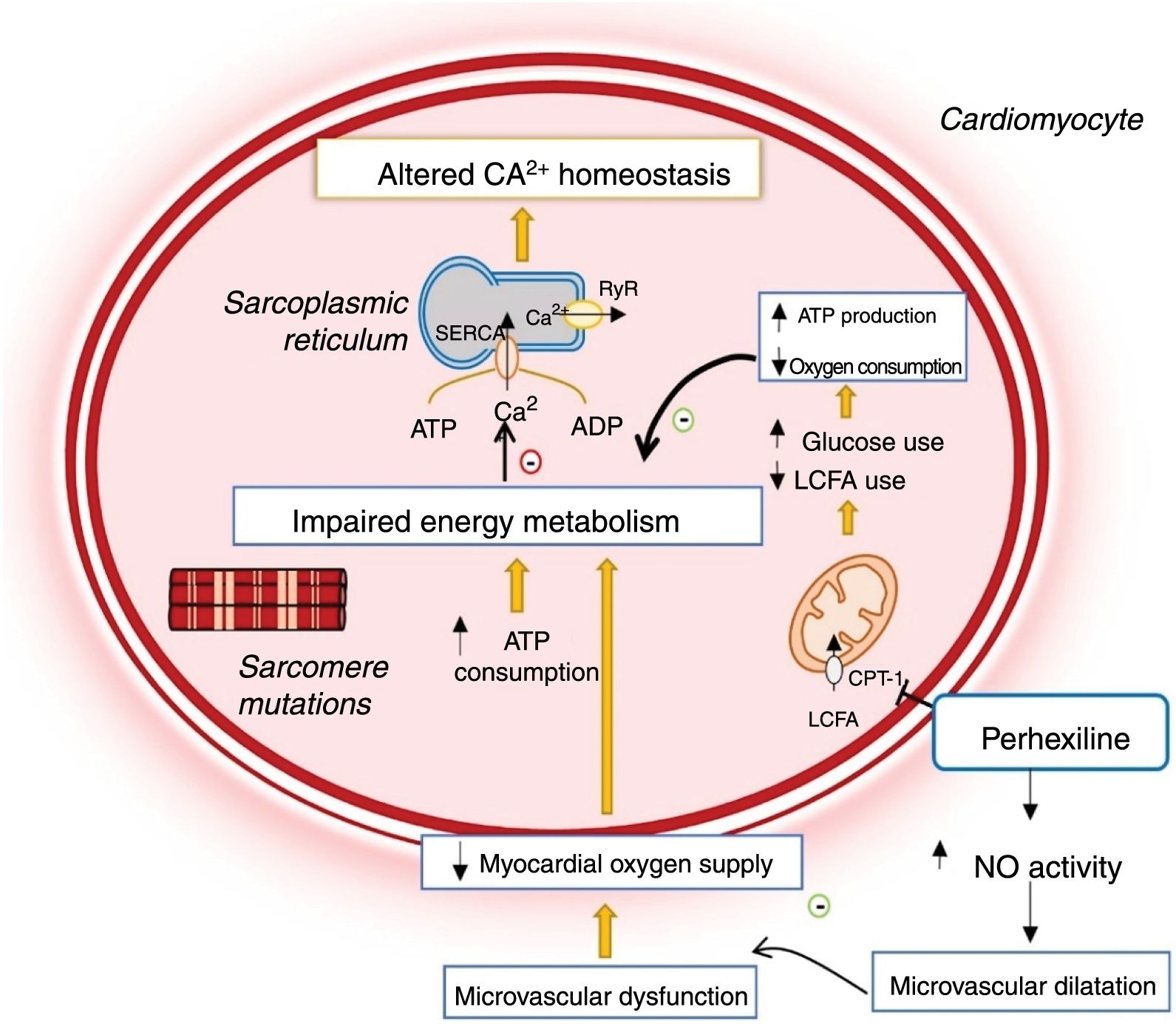
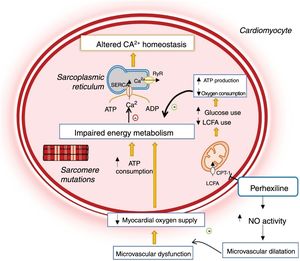
Changes in cardiomyocyte energy metabolism in hypertrophic cardiomyopathy and mechanism of action of perhexiline.12,14,24 ADP: adenosine diphosphate; ATP: adenosine triphosphate; CPT-1: carnitine-palmitoyl transferase 1; LCFA: long-chain fatty acids; NO: nitric oxide; RyR: ryanodine receptor; SERCA: sarco/endoplasmic reticulum Ca2+-ATPase.
Animal models of HCM suggest that treatment with calcium channel blockers may correct the dysregulation of calcium homeostasis by reducing influx into cardiomyocytes. In transgenic mice with the R403Q mutation in the MHY6 gene, Ca2+ and calcium-binding protein levels in the sarcoplasmic reticulum were reduced. This reduction precedes the alterations in myocardial morphology and histology typical of HCM.17 In this mouse model study, diltiazem reversed changes in Ca2+ homeostasis and prevented the development of LVH and interstitial fibrosis.17 It was subsequently demonstrated that diltiazem also prevented the development of diastolic dysfunction and sudden death in mouse carriers of the cardiac troponin T I79N mutation.19
In light of these results, diltiazem has been proposed as a disease-modifying treatment in HCM carriers who have not yet developed LVH.8,13 In a double-blind, randomized, placebo-controlled, pilot clinical trial of 38 carriers of sarcomere mutations without LVH,16 diltiazem (360 mg/day for 25 months) did not reduce maximum left ventricular (LV) wall thickness, but stabilized LV end-diastolic diameter and consequently the LV thickness to end-diastolic diameter ratio. In controls, this parameter decreased throughout the study period. In addition, the degree of pharmacological benefit appeared to depend on the specific sarcomere mutation; this was more evident among carriers of MYBPC3 mutations. As HCM is associated with reduced LV cavity size, the stabilization of LV end-diastolic diameter by diltiazem may reflect the beneficial effect of the drug on the disease's natural history. Diltiazem may therefore be useful in preventing the phenotypic expression of HCM in at-risk mutation carriers. However, the small sample and limited follow-up are major limitations of this trial.16
Late sodium current blockersLate sodium current (INaL) blockers include ranolazine, which can be used as a second-line treatment for symptom relief and improved functional capacity in stable coronary heart disease patients.
Disruption of intracellular sodium homeostasis may contribute to the pathophysiology of HCM,13,20,21 as raised sodium levels in the cardiomyocyte worsen the dysregulation of intracellular Ca2+ homeostasis which, as mentioned previously, is a key feature of the disease's pathogenesis (Figure 1).12,14–16
A recent study in cardiomyocytes and trabeculae isolated from the interventricular septum of HCM patients identified an increase in INaL amplitude in these cells and, consequently, a rise in intracellular Na+ levels.20 This change then leads to an increase in Ca2+ influx to the cell via the sodium-calcium exchanger (NCX), thus raising intracellular Ca2+ levels.21 Inhibition of INaL by ranolazine was shown to reduce intracellular sodium levels and hence Ca2+ influx and to re-establish homeostasis in cardiomyocytes (Figure 1). Together, these effects decreased the risk of arrhythmias and improved diastolic function. Prolonged INaL inhibition may eventually reduce the activation of signaling pathways that lead to adverse myocardial remodeling, with implications for disease progression.21
Ranolazine was thus thought to have the potential to modify the disease's natural history or to provide symptomatic relief. This hypothesis was the basis of the Ranolazine in Patients with Symptomatic Hypertrophic Cardiomyopathy (RESTYLE-HCM) trial, which assessed the beneficial effects of ranolazine on the functional capacity of HCM patients.22 In this double-blind, randomized, placebo-controlled trial in a sample of 80 symptomatic patients, although ranolazine reduced the risk of arrhythmia, it did not significantly improve LV diastolic function, exercise tolerance, quality of life or plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) levels.22
The Effect of Eleclazine (GS-6615) on Exercise Capacity in Subjects With Symptomatic Hypertrophic Cardiomyopathy (LIBERTY-HCM) trial assessed the effect of a more potent INaL inhibitor, eleclazine, on improving exercise tolerance in HCM.23 This trial was ended prematurely due to the adverse effects the drug presented in parallel trials in patients with other heart disease.23
Metabolic modulatorsPerhexiline is a metabolic modulator used for relief of refractory angina in ischemic heart disease.24–27 Its main mechanism of action is potent inhibition of carnitine palmitoyltransferase-1 (CPT-1) and, to a lesser extent, of carnitine palmitoyltransferase-2 (CPT-2).24–26 It is a complex pharmacological agent that is potentially neurotoxic and hepatotoxic, and so long-term serum levels need to be monitored.24–26
CPT-1 and CPT-2 are key enzymes for mitochondrial long-chain fatty acid transport.24–27 Their inhibition by perhexiline alters cell metabolism, reducing the oxidation of fatty acids and favoring the use of carbohydrates as metabolic substrate, which in turn is associated with more efficient use of oxygen for myocardial ATP production (Figure 2).24–26 Additionally, the drug enhances nitric oxide activity, increasing oxygen supply to the myocardium (Figure 2).24
Perhexiline may, therefore, have beneficial effects in HCM since sarcomere mutations increase cross-bridge formation and energy expenditure during cardiomyocyte contraction, which results in inefficient use of ATP (Figure 2).12,14,18,23 These changes lead to impaired myocardial energy metabolism, which is reflected in a reduced phosphocreatine/ATP ratio, a good indicator of myocardial energy status.12,14,18,26,28 This deficiency precedes the development of LVH and suggests that impaired myocardial energy metabolism plays a major role in the disease's pathophysiology.28
In addition, HCM is associated with microvascular dysfunction together with decreased vasodilator response and myocardial oxygen supply, which further exacerbates the primary energy deficiency (Figure 2).29 As perhexiline enhances nitric oxide activity, this drug also relieves the microvascular dysfunction that accompanies HCM (Figure 2).14,24,29
Perhexiline may therefore be useful for treating HCM (Figure 2). A double-blind, randomized, controlled clinical trial was conducted in 46 patients with symptomatic HCM to test this hypothesis.29 In this trial, the administration of perhexiline led to improved myocardial energy status, demonstrated by an increase in the phosphocreatine/ATP ratio in the myocardium. This increase was associated with improvements in patients’ symptoms, oxygen consumption and diastolic function.29
Despite these promising initial results, a recent clinical trial in 35 patients with obstructive HCM was discontinued prematurely as perhexiline showed no demonstrable efficacy in symptom improvement and oxygen consumption in HCM patients (ClinicalTrials.gov identifier NCT02862600). Another trial to assess the efficacy of perhexiline in improving the functional capacity of patients with HCM (ClinicalTrials.gov identifier NCT02431221) was also canceled before the start of patient recruitment due to the lack of demonstrable efficacy in the preceding trial.
Very recently, trimetazidine, another metabolic modulator, was studied in an HCM trial.30 By inhibiting beta oxidation of fatty acids, this drug also has potential beneficial effects on cardiomyocyte energy metabolism.30 However, in a recent double-blind, randomized, placebo-controlled clinical trial, trimetazidine did not improve peak oxygen consumption or six-minute walk test distance in patients with symptomatic non-obstructive HCM.30
Overall, despite their theoretical beneficial effect on the pathophysiology of HCM, metabolic modulators have not demonstrated efficacy in improving symptoms for patients with the disease. No other clinical trials on these drugs are currently underway due to reduced interest in their use following the disappointing results in the above studies.
Nevertheless, a small phase I pilot clinical trial to assess the effect of sodium nitrate on myocardial energy status (assessed by phosphocreatine/ATP ratio) in patients with HCM is currently in the recruitment phase (ClinicalTrials.gov identifier NCT03251287).
N-acetylcysteineN-acetylcysteine (NAC) is used as an antidote to acetaminophen poisoning,31 for which it supplies the cysteine required to re-establish levels of intracellular glutathione, an important antioxidant.31,32
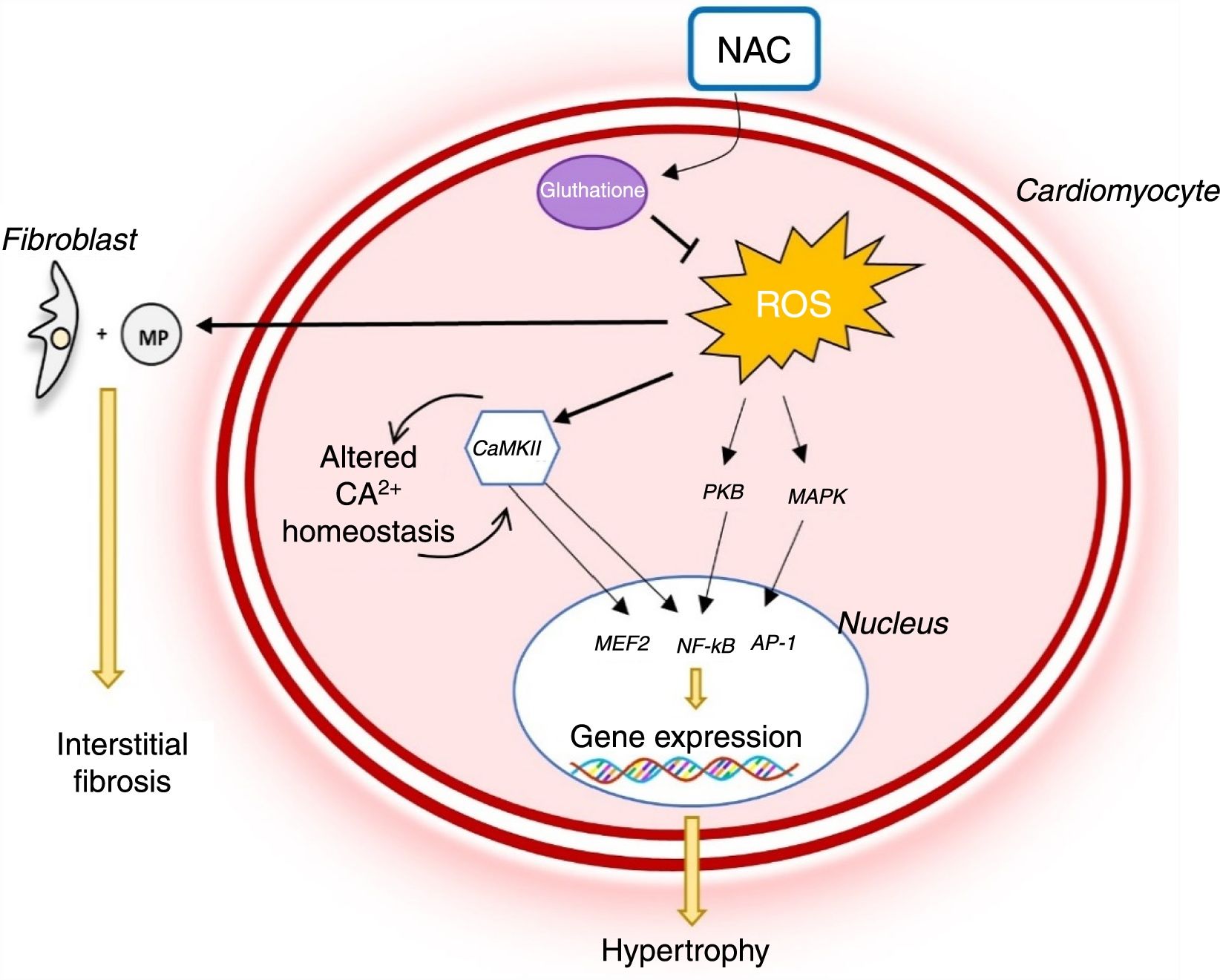
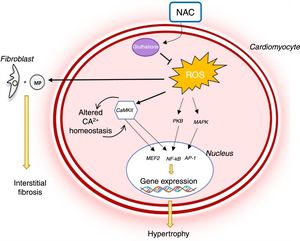
Reactive oxygen species (ROS) levels are known to rise in HCM and oxidative stress plays a major role in the pathophysiology of the disease (Figure 3). In rabbit models with the R403Q mutation in MYH7, the development of LVH and myocardial fibrosis was accompanied by an increase in levels of several oxidative stress markers.33 Oxidative stress contributes to the HCM phenotype by stimulating and activating hypertrophy-inducing signaling kinases and transcription factors.34 It also triggers fibroblast proliferation and activation of metalloproteinases, which promote interstitial fibrosis and extracellular matrix remodeling.35 Oxidative stress also leads to cardiomyocyte apoptosis, which contributes to myocardial dysfunction and remodeling (Figure 3).34
Role of oxidative stress in the pathophysiology of hypertrophic cardiomyopathy and mechanism of action of N-acetylcysteine.14,34 AP-1: activator protein; CaMKII: Ca2+/calmodulin-dependent protein kinase II; MAPK: mitogen-activated protein kinase; NAC: N-acetylcysteine; NF-κB: nuclear factor kappa B; PKB: protein kinase B; ROS: reactive oxygen species.
The antioxidant effect of NAC may thus play an important role in the treatment of HCM (Figure 3). In studies in animal models of HCM (mice with the R92Q mutation in the gene encoding troponin T), NAC reduced myocardial oxidative stress and interstitial collagen levels, partially reversing established fibrosis.35 In addition, it has been demonstrated that NAC re-establishes intracellular glutathione reserves, reverses established LVH and interstitial fibrosis, improves systolic function and reduces arrhythmogenic risk in rabbit carriers of the R403Q mutation in the gene encoding beta-myosin heavy chain.32
These promising results formed the basis for the Hypertrophy Regression With N-Acetylcysteine in Hypertrophic Cardiomyopathy (HALT-HCM) trial, a recent double-blind, randomized, placebo-controlled pilot trial to assess the effect of NAC on reversing LVH and interstitial fibrosis in HCM.36 In this study, the benefit of treatment with NAC on LVH and myocardial fibrosis was disappointingly small.36 The small sample does not, however, allow firm conclusions to be drawn on the efficacy of NAC in HCM.36
StatinsStatins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, an essential enzyme in cholesterol biosynthesis.33,37–40 Usually prescribed for dyslipidemia, statins have wide-ranging biological activity other than their effects on cholesterol levels.33,37–40 They have pleiotropic effects on oxidative stress, cell proliferation, endothelial function, coagulation and immunomodulation.33,37–40
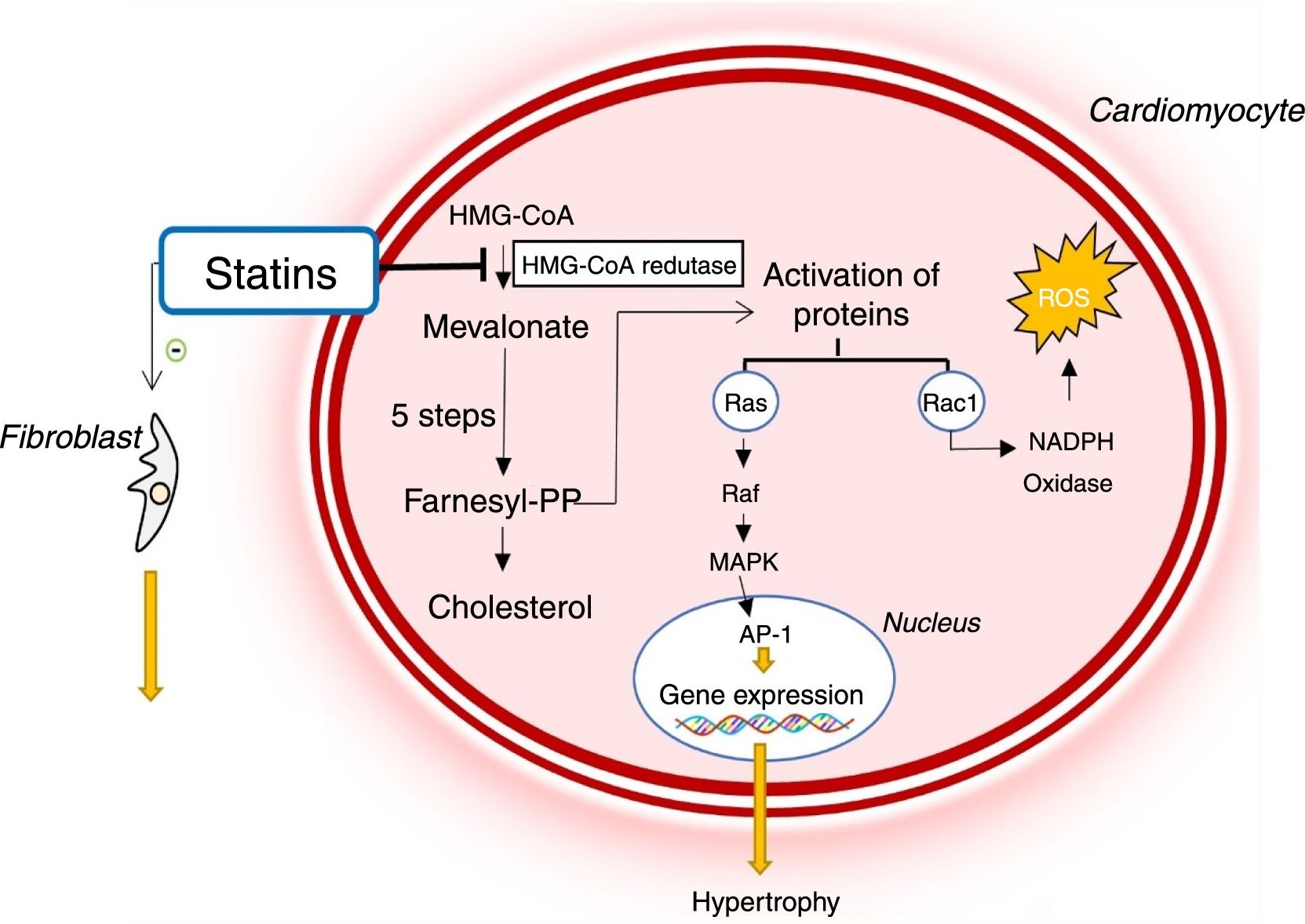
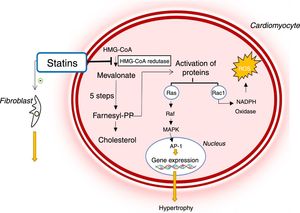
Due to these biological actions, statins are potentially beneficial in diseases such as HCM (Figure 5).37,39 By inhibiting HMG-CoA reductase, they reduce the activation of various signaling molecules that promote hypertrophy and cardiac interstitial fibrosis.33,39–41 In addition, they reduce oxidative stress by decreasing the Rac1-mediated generation of ROS, which contribute to the development of LVH and interstitial fibrosis (Figure 4).33,39–41
Mechanism of action of statins and their role in the pathophysiology of hypertrophic cardiomyopathy. AP-1: activator protein; Farnesyl-PP: farnesyl pyrophosphate; HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A reductase; MAPK: mitogen-activated protein kinase; NADP: nicotinamide adenine dinucleotide phosphate, ROS: reactive oxygen species.
In rabbit models of HCM with the R403Q mutation in the gene encoding beta-myosin heavy chain and established LVH, simvastatin improved diastolic function and reduced LV wall thickness and interstitial collagen levels, thus reversing cardiac hypertrophy and fibrosis.38 Another study using the same animal HCM model showed that administration of atorvastatin before the development of LVH reduces myocardial oxidative stress and prevents the development of LVH.33
By contrast, trials in humans have not shown a beneficial effect of statins in reverting the HCM phenotype. In a double-blind, randomized, placebo-controlled pilot clinical trial in 28 patients with HCM and established LVH, treatment with atorvastatin was not associated with decreased LV mass or improved diastolic function.42 This result was replicated in another trial with an initial sample of 21 patients, in which atorvastatin did not lead to LVH regression.41
The Statin Induced Regression of Cardiomyopathy Trial (SIRCAT) also assessed atorvastatin's potential to reverse LVH in patients with HCM.43 In this randomized, placebo-controlled clinical trial, atorvastatin was not shown to reduce LV mass compared with placebo.43
This divergence in results may be explained by the small samples used in the human trials, combined with the multiple mutations and pathophysiological pathways involved in HCM in humans, unlike the above animal models, all of which studied the mutation in the beta-myosin heavy chain. Even so, of the three clinical trials with atorvastatin in HCM that have been completed, none showed that this drug provides any benefit in reversing the HCM phenotype. It is therefore unlikely that atorvastatin can be used as a disease-modifying therapy in HCM. Nevertheless, it is possible that other statins may be effective in preventing or reversing LVH and interstitial fibrosis.
Renin-angiotensin-aldosterone system inhibitorsRenin-angiotensin-aldosterone system (RAAS) inhibitors such as ACE inhibitors, ARBs and MRAs can be used in combination with beta-blockers to treat HCM-related heart failure with ejection fraction <50% and without LVOT obstruction.1
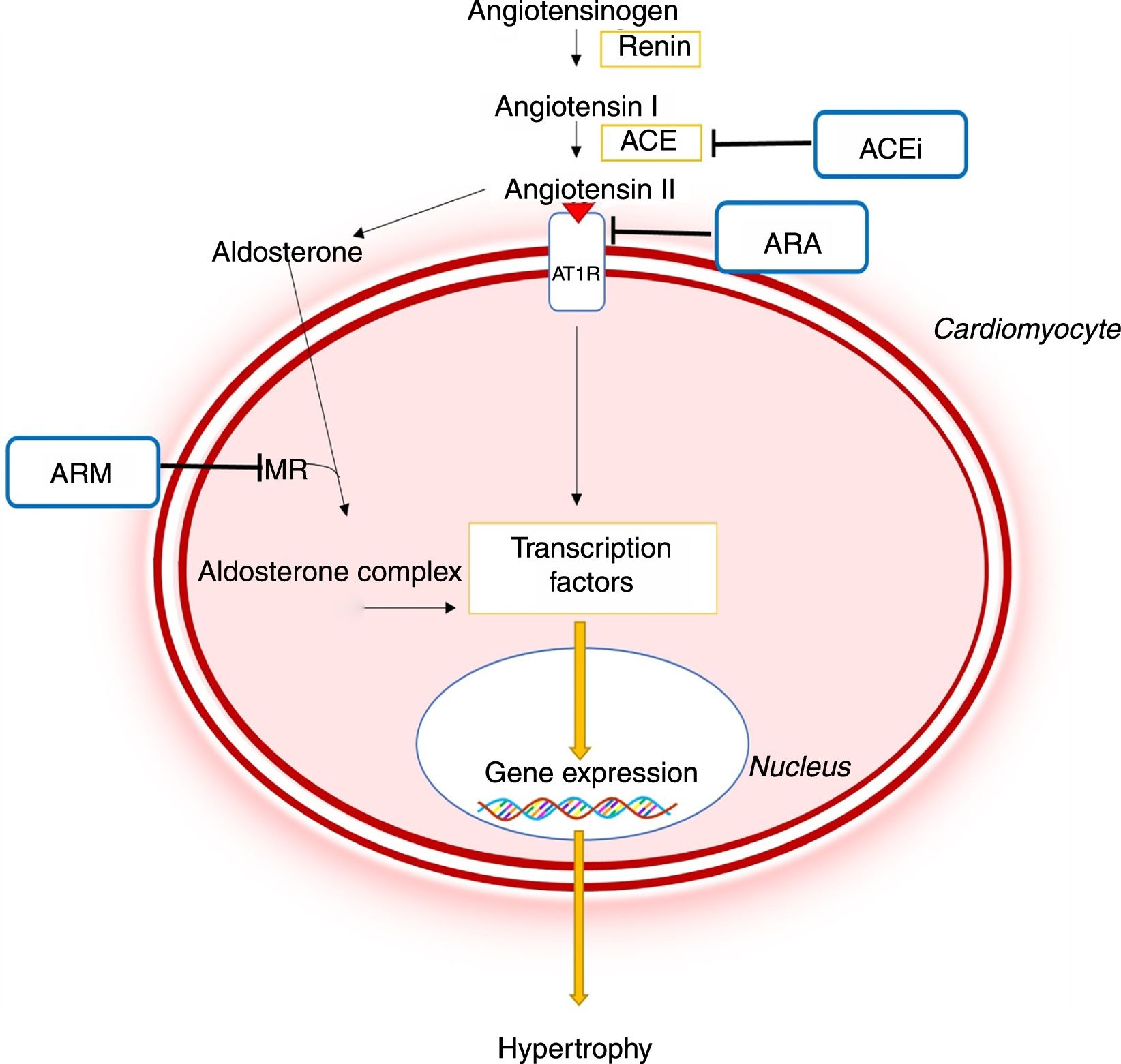
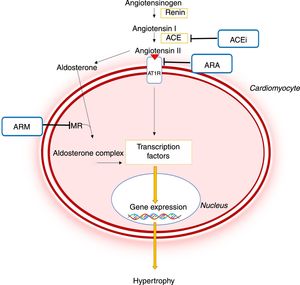
As in other conditions like hypertension, in which angiotensin II and aldosterone promote adverse cardiac and vascular remodeling, it is now recognized that RAAS activation plays a major role in the pathophysiology of HCM (Figure 5).8,44–46 This system contributes to the development of LVH and myocardial fibrosis through profibrotic and prohypertrophic effects mediated by circulating angiotensin II and by local RAAS activation in the myocardium.44–46 In addition, it has been demonstrated that polymorphisms in genes encoding ACE or the angiotensin II receptor type I may lead to a greater degree of LVH in patients with HCM.45
Importance of the renin-angiotensin-aldosterone system in the pathophysiology of hypertrophic cardiomyopathy and mechanism of action of different pharmacological inhibitors of this system.44,45 ACE: angiotensin-converting enzyme; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; AT1R: angiotensin II receptor type I; CaMKII: Ca2+/calmodulin-dependent protein kinase II; JAK: Janus kinase; MEF2: myocyte enhancer factor 2; MRA: mineralocorticoid receptor antagonist; NF-κB: nuclear factor kappa B; MR: mineralocorticoid receptor; STAT: signal transducer and activator of transcription.
Pharmacological inhibition of the RAAS may therefore help prevent or reverse the HCM phenotype (Figure 5).7,8 Mice models with the R92Q mutation in the troponin T gene have shown that the ARB losartan reduces the levels of transforming growth factor beta-1, a profibrotic mediator of angiotensin II, and reduces interstitial collagen levels in the heart.47 The MRA spironolactone also reversed interstitial fibrosis and cardiomyocyte disarray in the same animal model, as well as improving diastolic function.44 In another animal model of HCM – SS-16BN/Mcwi consomic rats – treatment with the ACE inhibitor captopril reduced LVH development.48 Captopril combined with spironolactone also reduced LV wall thickness more significantly than monotherapy with captopril.48
The results from the first trials in humans were also promising. Administration of the ARB valsartan in a randomized, non-placebo-controlled clinical trial in 23 individuals with HCM reduced the synthesis of collagen type I, one of the main types of collagen in the myocardium.49 In a non-controlled, non-randomized, pilot clinical trial, losartan, in addition to the patients’ usual therapy, was associated with reduced symptoms, improved LV diastolic function and reduced NT-proBNP levels.50
These first trials did not demonstrate that ARBs reversed established LVH in human subjects.49,50 More recent studies, however, suggest that these drugs may in fact have this beneficial effect. In a double-blind, randomized, non-placebo-controlled pilot clinical trial, losartan resulted in a slight decrease in LV mass.51 This was replicated in another randomized, placebo-controlled trial with a similar sample of 20 patients with HCM.52 In this trial, losartan also reduced the degree of myocardial interstitial fibrosis, as demonstrated by a reduction in late gadolinium enhancement (LGE) on CMR imaging.52
In a third double-blind, randomized, placebo-controlled clinical trial in 24 patients with HCM and LVH, the ARB candesartan also reversed LVH and led to improvements in ventricular function parameters as assessed by echocardiography, symptoms and exercise tolerance.53 In this trial, the magnitude of benefit achieved was dependent on the specific sarcomere mutation involved and was more marked in patients with mutations in the MYH7 gene.53
The Inhibition of the Renin Angiotensin System With Losartan in Patients With Hypertrophic Cardiomyopathy (INHERIT) trial was conducted to shed more light on the conflicting results of these clinical trials and to establish the efficacy of RAAS inhibitors in reversing LVH and interstitial fibrosis in HCM.54 In this double-blind, placebo-controlled, randomized trial in 124 patients, treatment with losartan, although safe, did not significantly reduce LV mass or interstitial fibrosis.54 This result does not support the hypothesis that this agent can change the HCM phenotype in individuals with established disease.54
Nevertheless, RAAS inhibition may still be useful for preventing disease development in patients with preclinical HCM (genotype-positive, phenotype-negative patients).54 The Valsartan for Attenuating Disease Evolution In Early Sarcomeric HCM (VANISH) trial is currently under way in a desirable sample of 150 carriers of HCM-related mutations but without LVH. This trial assesses the efficacy of valsartan therapy in preventing the phenotypic expression of this disease.7,23
In addition, a clinical trial is under way to assess the effect of spironolactone in reducing myocardial fibrosis, as determined by LGE on CMR (ClinicalTrials.gov identifier NCT02948998).
Sarcomere contractility modulatorsMyosin is one of the proteins of the sarcomere contractile unit. It is an adenosine triphosphatase (ATPase) that interacts cyclically with actin filaments to convert energy into force that powers cardiomyocyte contraction 55
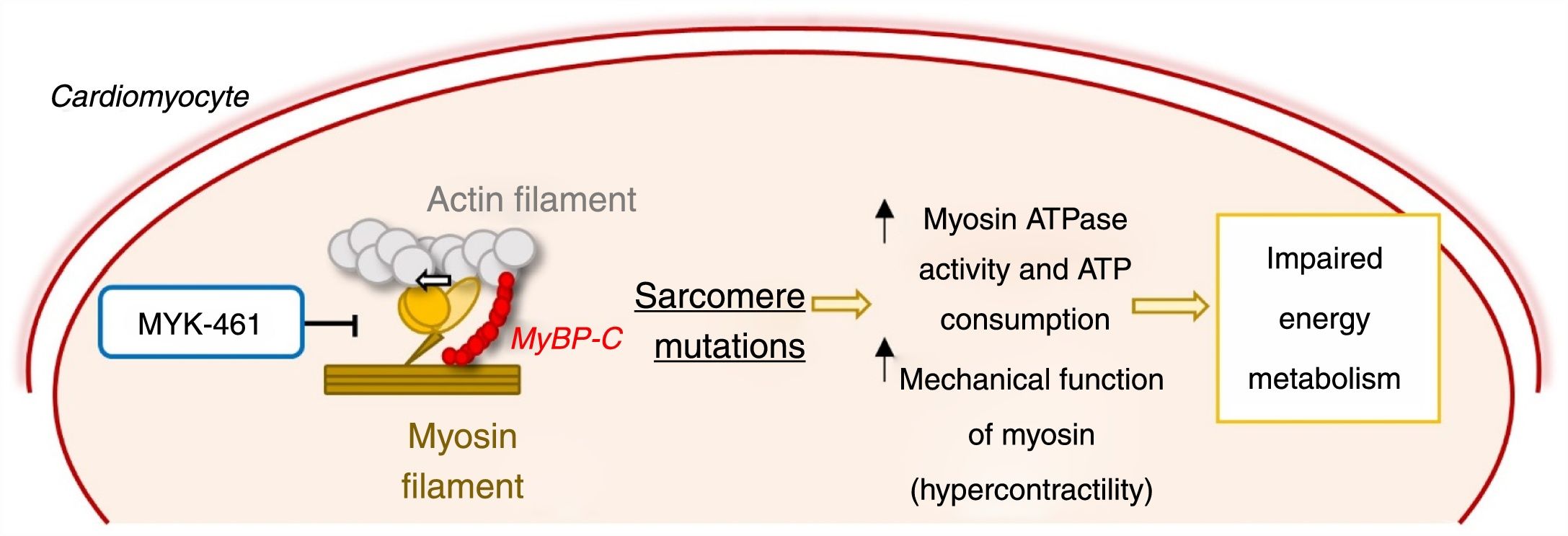
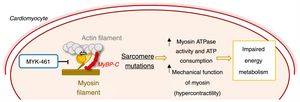
As stated above, HCM is mainly caused by sarcomere protein mutations, most commonly in the MYH7 and MYBPC3 genes.1–6 It was shown that mutations in MYH7 lead to a state of hypercontractility in which the ATPase activity and mechanical function (force and motion generation) of cardiac myosin are increased.55 This gain of function leads to increased consumption of ATP, which in turn contributes to impaired energy metabolism, promoting disease development (Figure 6).18,55
Mechanism of action of the allosteric myosin inhibitor MYK-461/mavacamten and its effect in the pathophysiology of hypertrophic cardiomyopathy.55,56 ATP: adenosine triphosphate; MyBPC: myosin-binding protein C.
Agents that reduce this increase in myosin activity may prevent the development of HCM. Mavacamten (MYK-461), an allosteric myosin inhibitor that reduces its ATPase activity and consequently the force generation and contractility of the sarcomere, was recently developed to test this hypothesis (Figure 6).56 Administration of MYK-461 in animal models of HCM prevented the development of LVH in prehypertrophic mice and partially reversed hypertrophy in mice with the established phenotype. Histopathologically, this molecule prevented (but did not reverse) the development of myocardial fibrosis and cardiomyocyte disarray. In addition, it reduced the expression of profibrotic and prohypertrophic genes.56
The safety of MYK-461 has been demonstrated in small phase I clinical trials in healthy individuals and those with established HCM.23 PIONEER-HCM (A Phase 2 Open-label Pilot Study Evaluating MYK-461 in Subjects With Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction) was recently completed in 21 patients with established obstructive HCM. It showed that in the high-dose arm (the results from the other arm have not been published), MYK-461 significantly reduced peak LVOT gradient following exercise and improved peak oxygen consumption.23
In addition, the MAVERICK-HCM clinical trial (A Phase 2 Study of Mavacamten in Adults With Symptomatic Non-Obstructive Hypertrophic Cardiomyopathy), currently under way, aims to assess the safety and tolerability of mavacamten in patients with non-obstructive HCM, while the Clinical Study to Evaluate Mavacamten (MYK-461) in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy (EXPLORER-HCM), a trial with a desirable sample of 250 patients, aims to assess the effect of MYK-461 on peak oxygen consumption in patients with obstructive HCM.23
Gene therapyGene therapy has the potential to help prevent disease development in HCM.14,57
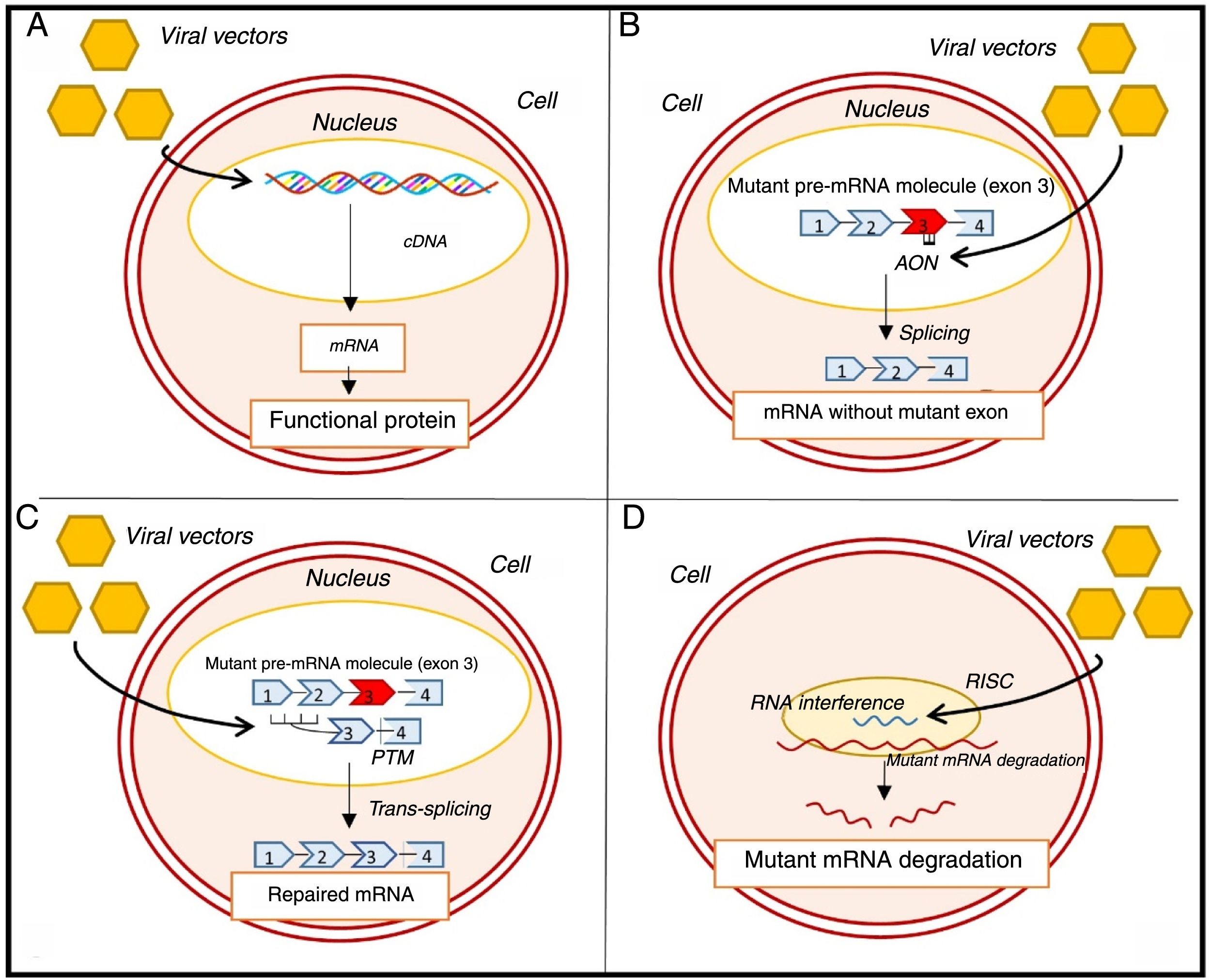
Recently, efforts have been made to correct HCM by inducing the expression of a functional sarcomere protein which replaces the endogenous mutated form (Figure 7A).14,57 This strategy is of particular interest in MYBPC3 mutations, which are associated with low levels of the sarcomere protein (haploinsufficiency).14,57 In a recent study, the transfer, using viral vectors, of wild-type MYBPC3 complementary deoxyribonucleic acid (cDNA) to mice with a mutation in this gene led to a dose-dependent increase in levels of messenger ribonucleic acid (mRNA) and wild-type myosin-binding protein C.58 It also suppressed mutant mRNA levels and prevented long-term development of LVH and systolic and diastolic dysfunction.58 Another study supported these results and showed that cardiomyocytes with a mutation in the MYBPC3 gene, derived from induced pluripotent stem cells isolated from an HCM patient, presented hypertrophy and reduced levels of myosin-binding protein C.59 In this study, the transfer of wild-type MYBPC3 cDNA increased the levels of this protein, which reversed cardiomyocyte hypertrophy.59
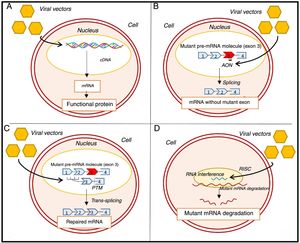
Genetic approaches to the treatment of hypertrophic cardiomyopathy. (A) Gene transfer; (B) exon skipping; (C) SMaRT technique; (D) allele silencing with RNAi.57–60,63,65 AON: antisense oligonucleotides; cDNA: complementary deoxyribonucleic acid; mRNA: messenger ribonucleic acid; pre-mRNA: precursor messenger ribonucleic acid; PTM: Pre-trans-splicing molecule; RISC: RNA-induced silencing complex.
Other equally promising approaches aim to alter mRNA. One technique under consideration involves exon skipping, in which antisense oligonucleotides are introduced through viral vectors (Figure 7B).14,57,60,61 These oligonucleotides are complementary to a specific region of interest in precursor mRNA (pre-mRNA), and prevent the binding of splicing-regulatory proteins in this region, preventing the inclusion of target exons in mature mRNA.14,57,60,61 A recent proof-of-concept study showed that in mice with MYBPC3 mutations, the use of this technique to remove the exon in which the mutation was found increased the levels of a functional isoform of myosin-binding protein C.60 In addition, it prevented the development of systolic dysfunction and LVH in these mice, although this effect was transient.60
Another strategy involves the repair of mutated pre-mRNA using spliceosome-mediated RNA trans-splicing (SMaRT) (Figure 7C).14,57,61,62,65 In this technique, two molecules – the mutant pre-mRNA and the therapeutic molecule, delivered to the cell via a viral vector – are spliced together to create a repaired mRNA molecule.57,62,65 In animal models with an MYBPC3 mutation, the SMaRT technique corrected the altered mRNA but was not associated with a significant increase in normal protein levels or prevention of the HCM phenotype.63 The low efficiency of the SMaRT technique was also observed in a study of induced pluripotent stem cell-derived cardiomyocytes.59
Another approach involves RNA interference (RNAi) using viral vectors to silence HCM-associated mutations.14,65 In a study of mice with a mutation in the MHY6 gene, this technique prevented the development of the disease phenotype (Figure 7D).64
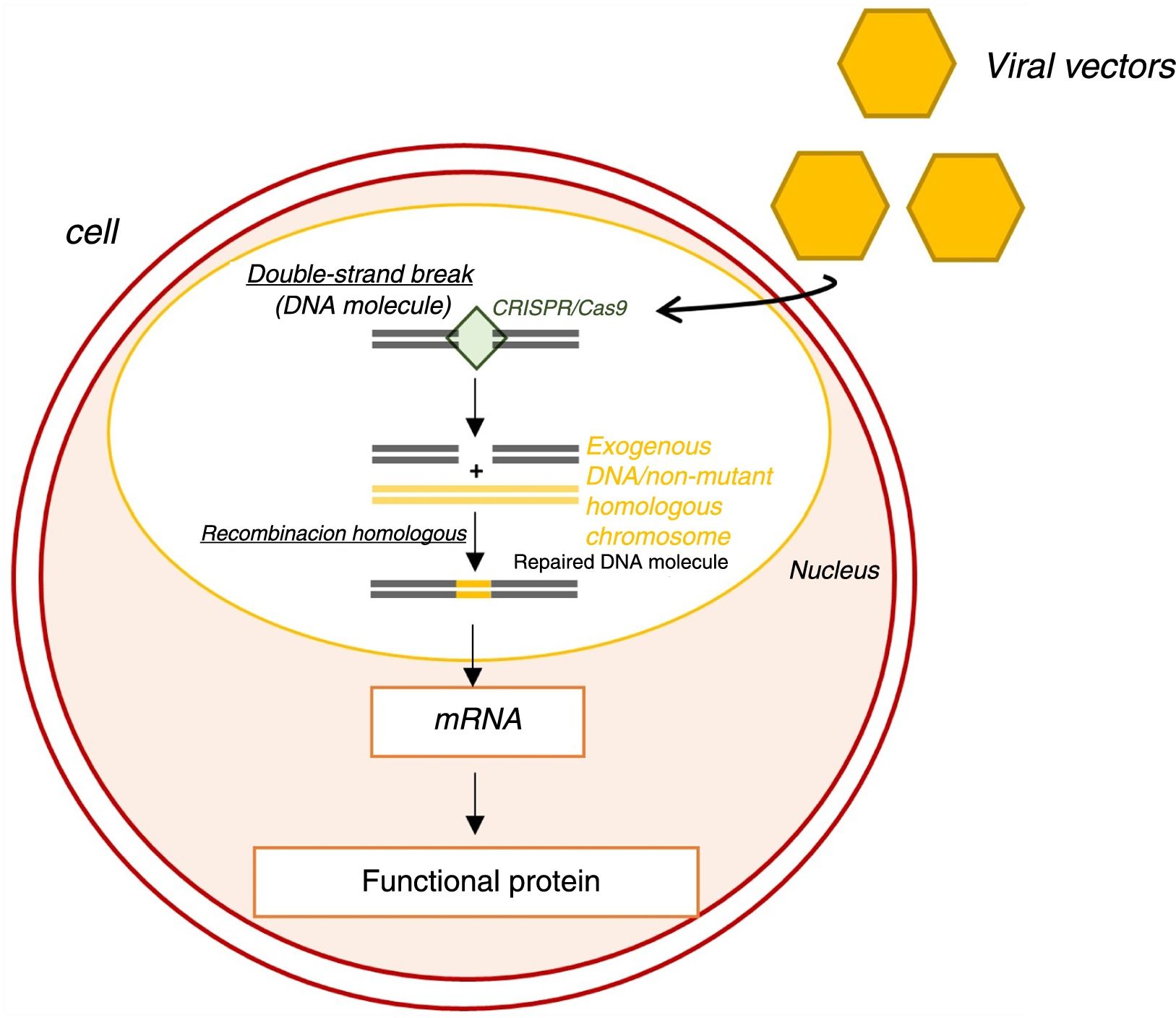
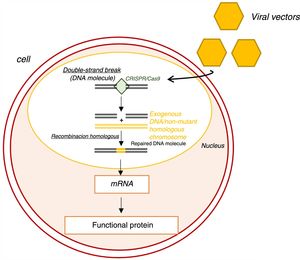
Genome editing using the clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9 (CRISPR/Cas9) system is also being investigated.65 Functioning as a nuclease, this system induces double-strand breaks in DNA (Figure 8).65 These breaks are then repaired by endogenous molecular mechanisms, generally by homologous recombination, which corrects the mutant allele without inducing undesired new mutations.65
Genome editing with the clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9 (CRISPR/Cas9) system.65 CRISPR-Cas9: clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9; mRNA: messenger ribonucleic acid.
A recent study sought to correct a mutation in the MYBPC3 gene in stem cells and human embryos using CRISPR/Cas9.66 In this study, the efficiency of genome editing was much higher in embryos than in stem cells.66 In embryos it was often corrected by homologous recombination (using the unmutated homologous chromosome), which, as stated above, has a lower risk of inducing new mutations.66 These results were not associated with effects in undesired genes or changes in embryo development induced by CRISPR/Cas9 and, by modulating the cell cycle stage at which the double-chain break was induced, the creation of mosaicisms was avoided.66 High efficiency in genome editing and the lack of side effects suggest that the CRISPR/Cas9 system has the potential to correct hereditary mutations in human embryos.66
ConclusionAlthough for many decades considered a rare disease, HCM is now known to be relatively common, with potentially harmful consequences for affected individuals. Despite this, treatment has changed very little since the disease was first described. The search for approaches that revert or prevent the disease phenotype, and consequently its symptoms and complications, remains challenging. However, substantial advances in knowledge of HCM pathophysiology have enabled the development of therapies with the potential to satisfy the unmet need in HCM treatment.
These approaches are highly attractive: different drugs and strategies targeting specific sarcomere mutations may be used, including modulating sarcomere protein contractility and gene therapy. Most of them have been shown in studies of animal models to potentially act as disease-modifying therapies. Positive results have been obtained for some drugs and molecules, including diltiazem and MYK-461/mavacamten, in initial clinical trials in humans, and new studies are expected in the future to test their efficacy. Other drugs such as ranolazine, perhexiline, NAC, RAAS inhibitors and statins have presented disappointing or conflicting results in trials in humans, and additional trials on some of these drugs are awaited to determine their efficacy (or lack of efficacy) in the treatment of HCM.
Gene therapy is a highly promising strategy due to its ability to act on the earliest changes in the pathophysiology of HCM. This technique is, however, in its very early stages and no trials have yet been conducted in human subjects.
These approaches are targeted at key events early in the development of HCM. Unlike the drugs currently recommended for the treatment of HCM, which seek to alleviate symptoms or prevent complications, these new strategies may mitigate or delay the development of the disease phenotype or even prevent disease onset. This is particularly promising for carriers of disease-associated mutations without phenotype expression (genotype-positive, phenotype-negative individuals), in whom preventing the development of LVH or other disease-associated alterations means the disease may never take hold. Even in individuals with established disease, the possibility of stabilizing the phenotype using the therapeutic strategies described in this article is of major interest and importance. However, it is also important to note that in carriers of late-onset mutations, the effects of these modifying therapies will be more difficult to ascertain.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Maltês S, Lopes LR. Novas perspetivas no tratamento farmacológico da miocardiopatia hipertrófica. Rev Port Cardiol. 2020;39:99–109.