An 80-year-old woman with rheumatic valve disease and two previous cardiac surgeries was admitted for heart failure exacerbation. The patient presented stenotic aortic 19-mm Mitroflow and mitral 31-mm Carpentier-Edwards bioprostheses, and was deemed inoperable due to frailty and prohibitive surgical risk. The heart team decided on a compassionate double valve-in-valve procedure, with transfemoral implantation of a 23-mm aortic CoreValve Evolut R and transapical implantation of a 29-mm mitral Edwards SAPIEN 3. During the procedure, after extreme difficulty in retrograde crossing of the aortic valve, a transapical-transfemoral loop was successfully performed. The procedure was without complications and the patient was discharged in NYHA class II with normally functioning valves.
Uma mulher de 80 anos com valvulopatia reumática e duas cirurgias cardíacas prévias foi internada por insuficiência cardíaca agudizada. A doente apresentava estenose de biopróteses Mitroflow 19 em posição aórtica e Carpentier-Edwards 31 em posição mitral, sendo considerada inoperável devido a fragilidade e a risco cirúrgico proibitivo. A Heart Team decidiu por um duplo procedimento valve-in-valve compassionate: implantação de Corevalve Evolut R 23 em posição aórtica via transfemoral e de Edwards Sapiens 3 29 em posição mitral por via apical. Durante o procedimento e após dificuldades extremas na progressão por via retrógrada aórtica, uma ansa transapical-femoral foi bem-sucedida. O procedimento correu sem complicações. A doente teve alta em classe NYHA II com biopróteses normofuncionantes.
Although surgical valve replacement has been the standard of care for several decades, transcatheter aortic valve implantation (TAVI) is currently a viable option for patients with severe aortic stenosis at intermediate and high surgical risk.1,2 Experience with TAVI in valve-in-valve procedures due to degenerated surgical bioprostheses is also increasing in patients deemed inoperable.2 Mitroflow surgical bioprostheses (Sorin S.p.A., Milan, Italy) present an increased incidence of coronary obstruction due to the shorter distance from the leaflets to the coronary ostia.3
Case reportAn 80-year-old woman with rheumatic valve disease and two previous cardiac surgeries was admitted for exacerbation of chronic heart failure. The patient's first cardiac surgery was in 1974 (mitral valvuloplasty) followed in 2006 by implantation of a 19-mm Mitroflow bioprosthesis in aortic position, a 31-mm Carpentier-Edwards (Edwards Lifesciences, Irvine, CA) bioprosthesis in mitral position, and a 31-mm Edwards annuloplasty ring (Edwards Lifesciences, Irvine, CA) in tricuspid position.
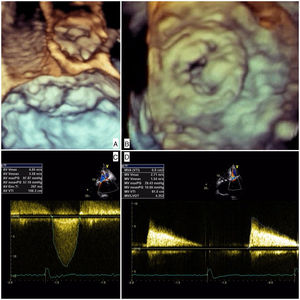
The patient was admitted with dyspnea and marked limitation of physical activity. Baseline serum creatinine was 0.7 mg/dl (estimated glomerular filtration rate [eGFR] 43 ml/min/1.73 m2 by the Cockcroft-Gault equation) and N-terminal pro-brain natriuretic peptide 18 432 pg/ml. The electrocardiogram showed atrial fibrillation at 90 bpm. Transthoracic echocardiography (TTE) showed severe stenosis of the Mitroflow (maximum gradient 98 mmHg, mean gradient 57 mmHg, aortic valve area 0.4 cm2) and severe stenosis of the Carpentier-Edwards (maximum gradient 29 mmHg, mean gradient 11 mmHg, mitral valve area 0.6 cm2) due to thick, almost immobile leaflets (Figure 1 and Video 1]. Coronary angiography showed anomalous left coronary origin of the right coronary artery and no significant lesions. The patient was deemed inoperable due to frailty (height 150 cm, weight 42 kg) and prohibitive surgical risk (Society of Thoracic Surgeons mortality risk score 22%). To further assess prosthesis sizes and vascular anatomy, multislice computed tomography angiography was performed, which revealed a moderate risk of occlusion of the left main with a distance of 7.7 mm from the valve apparatus to the ostium origin. The aortic annulus was small and non-circular (16 mm×18 mm, area 2.0 cm2) and the iliac and femoral arteries showed a minimal luminal diameter of 6.9 mm. The heart team decided on a compassionate double valve-in-valve procedure as the best therapeutic option for this patient, who consented to the intervention.
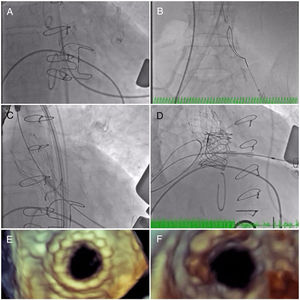
The initial plan was to implant a 23-mm CoreValve Evolut R (Medtronic, Irvine, CA) in aortic position (transfemoral approach) and a 29-mm Edwards SAPIEN 3 in mitral position (transapical approach). The procedure began with a successful left transfemoral approach with a 14-F Fast-Cath sheath, and the left coronary artery was protected with an angioplasty guidewire. Due to extreme difficulty in retrograde crossing of the aorta, a transapical-transfemoral loop approach was considered. The loop was created after gaining transapical access (Figure 2A), through which an INFINITI MPA 2 catheter (5.0 Fr×125 cm, Cordis) was used to advance an Emerald J wire (0.035″×260 cm) anterogradely until it reached the left common iliac artery, where it was captured with a 25-mm Amplatz Goose Neck snare (Figure 2B). After wire externalization at the left groin, the MPA 2 catheter was removed from the apical access and inserted via the groin, enabling the Emerald J wire to be exchanged for an Amplatz Extra Stiff guidewire (0.035″×260 cm) via a left transfemoral route (Video 2). The CoreValve was then successfully advanced into the aortic root and implanted in a suprannular aortic position under rapid pacing (Figure 2C and Video 3), 7 mm below the radiopaque Mitroflow ring. The left coronary ostium was unobstructed. Subsequently, the mitral valve-in-valve Edwards was successfully implanted transapically, without complications (Figure 2D and Video 4). Intraprocedural transesophageal echocardiography showed normal gradients (mean aortic gradient 11 mmHg, mean mitral gradient 3 mmHg) (Figure 2E and F) and a mild aortic perivalvular leak (Video 5). There were no immediate procedural complications. Due to contrast nephropathy and development of anuria (serum creatinine increased to 5.1 g/dl), the patient needed temporary renal replacement therapy, which was discontinued after two and a half weeks due to resumed diuresis. Before discharge, TTE revealed a small paravalvular aortic leak and normally functioning prosthetic valves. The patient was discharged in New York Heart Association (NYHA) functional class II on the 67th day with mild anemia (hemoglobin 11.5 g/dl), creatinine 1.0 mg/dl and eGFR 30 ml/min/1.73 m2.
(A) A long Emerald J wire passes through the stenotic Mitroflow, in which it is captured with an Amplatz Goose Neck snare (B); valve-in-valve implantation of a 23-mm CoreValve Evolut R in aortic position by the transfemoral route (C); implantation of a 29-mm Edwards SAPIEN 3 in mitral position by the transapical route (D); new aortic (E) and mitral (F) bioprosthetic valves visualized by transesophageal echocardiography during the procedure following implantation.
At 12 months of follow-up the patient was still in NYHA class II, with mean aortic gradient of 25 mmHg but a velocity time integral ratio of 0.53 and mean mitral gradient of 7 mmHg, probably due to mild anemia (hemoglobin 10 g/dl), without morphologic anomalies or new leaks.
DiscussionReports of valve-in-valve interventions are increasing in both number and complexity in the literature, with apparently favorable outcomes, supporting this approach as a viable option for selected patients.4,5 Our case depicts a new solution to cross the aortic valve and establish a stable transapical-transfemoral loop. This is an alternative in very small and severely degenerated bioprosthetic valves for which a retrograde approach may be difficult due to severe calcification and configuration, especially in the context of double valve-in-valve interventions. We also showed that while clinically challenging, the CoreValve Evolut is a good option for valve-in-valve implantation in the small diameter 19-mm Mitroflow valve in aortic position.
ConclusionWe report the first use of a transapical-transfemoral wire loop as part of a double valve-in-valve intervention. We believe that this technique may be a viable approach for TAVI procedures in very small stenotic bioprostheses. To our knowledge, this is the first reported case of a CoreValve Evolut implanted in a 19-mm Mitroflow aortic valve.
Conflicts of interestThe authors have no conflicts of interest to declare.
The following are the supplementary material to this article:
Transapical-transfemoral loop formation: a long 5 F wire was advanced until it reached the left common iliac artery, where it was captured with a snare. An INFINITI MPA 2 catheter (5 F×125 cm, Cordis, Johnson & Johnson, Fremont, CA) was introduced transapically for exchange with an Extra Stiff wire in the left femoral artery.